AP Syllabus focus:
‘Describe how monosaccharides, or simple sugars, act as monomers that bond covalently to form polysaccharides, or complex carbohydrates.’
Monosaccharides are the simplest carbohydrate units and serve as building blocks for larger molecules. Understanding how they covalently link into polysaccharides connects molecular structure to how organisms store energy and build carbohydrate-based materials.
Monosaccharides as Carbohydrate Monomers
What Monosaccharides Are
Monosaccharides are single sugar units that can be linked together to build larger carbohydrates. They commonly exist as rings in water, but their key role in this topic is that they function as monomers used to construct polysaccharides.
Monosaccharide: A simple sugar that functions as a single carbohydrate monomer; it can be covalently bonded to other monosaccharides to form larger carbohydrates.
Monosaccharides contain many hydroxyl (–OH) groups and one carbonyl group, making them reactive enough to form covalent bonds with other sugars.
Why Monosaccharides Link Together
Cells build carbohydrate polymers to create molecules with properties not found in single sugars, such as:
Energy storage capacity (many sugar units in one molecule)
Osmotic advantages (large polymers exert less osmotic pressure than many free monomers)
Structural potential (repeating units form stable carbohydrate frameworks)
Covalent Bonding to Form Polysaccharides
Polysaccharides as Complex Carbohydrates
A polysaccharide forms when many monosaccharides are joined into a chain or network by covalent bonds. The repeated monosaccharide subunits are often the same type, but the resulting polymer can differ greatly depending on how the units are connected.
Polysaccharide: A complex carbohydrate polymer made of many monosaccharide monomers covalently bonded together.
Because the covalent links are strong, polysaccharides are stable molecules that can be built up or broken down by enzymes when needed.
The Covalent Bond: Glycosidic Linkage
Monosaccharides join via a glycosidic linkage, a covalent bond formed between functional groups on two sugar molecules (typically involving hydroxyl groups). The specific atoms connected and the orientation of the bond help determine the polymer’s overall shape and properties.
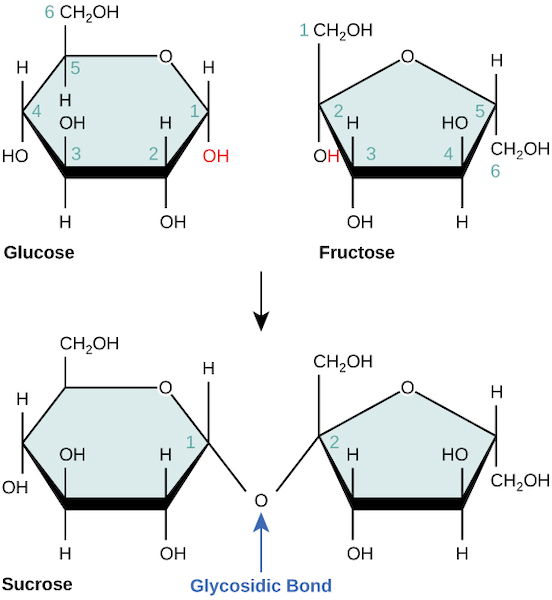
This figure depicts formation of a glycosidic bond between two monosaccharide rings (shown as glucose + fructose forming sucrose) and labels the bond directly. The carbon numbering emphasizes that which carbons participate in the linkage helps determine the resulting carbohydrate’s structure. Source
Glycosidic linkage: A covalent bond that joins two monosaccharides, formed when groups on the sugars react to connect them into a larger carbohydrate.
In biological systems, enzymes control which glycosidic linkages form, allowing cells to build specific polysaccharides with predictable structures.
Condensation (Dehydration) Reaction Overview
Formation of a glycosidic linkage typically occurs through a condensation reaction, in which:
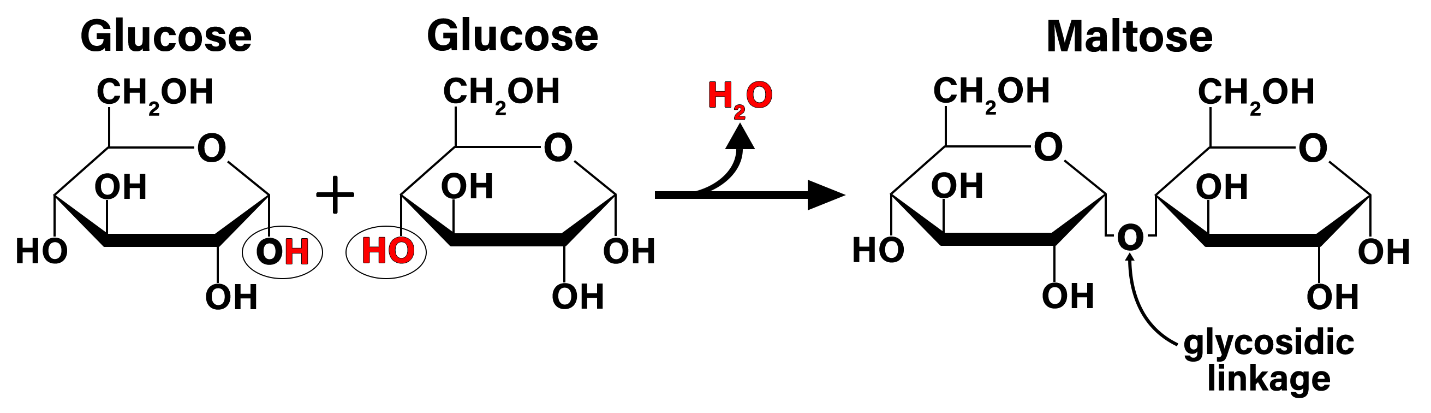
This diagram shows dehydration synthesis between two monosaccharides, highlighting the specific –OH and –H removed to form . The remaining atoms form a new covalent bond (a glycosidic linkage) connecting the sugar units into a larger carbohydrate. Source
A hydroxyl (–OH) from one monosaccharide and a hydrogen (–H) from another are removed
A molecule of water is produced
A new covalent bond forms between the sugars
This explains the syllabus emphasis that monosaccharides “bond covalently” to form polysaccharides.
How Structure Depends on the Monosaccharides and Their Connections
Monomer Identity and Linkage Patterns
Even when a polysaccharide is made from only one kind of monosaccharide, its properties can vary due to:
Which carbons are linked (for example, different carbon positions can be joined)
Bond orientation (the spatial arrangement of the glycosidic linkage)
Repetition and regularity (consistent linkages produce repeating patterns)
These structural features influence whether the polysaccharide tends to form more extended strands, more compact shapes, or interconnected networks.
Key Biological Implications (Kept at Formation Level)
At the level of formation, the essential idea is:
Monosaccharides are monomers
Polysaccharides are polymers
Covalent glycosidic linkages connect monomers into complex carbohydrates
Enzymes determine the specific linkages, which helps determine the polymer’s larger-scale structure
FAQ
In cells, many monosaccharides are predominantly in ring forms in aqueous solution, but they can interconvert between ring and open-chain forms.
Enzymes can still catalyse glycosidic bond formation because the relevant hydroxyl groups are available on the ring structure.
A reducing sugar has a free anomeric carbon that can participate in reactions that reduce other compounds.
When a glycosidic bond uses the anomeric carbon, that end may no longer be reducing; whether a polysaccharide has reducing ends depends on how its linkages are arranged.
Cells use specific enzymes that position monosaccharides and catalyse bond formation at particular carbon atoms and orientations.
This enzyme specificity ensures the same monomers can be assembled into different polysaccharides with distinct shapes and functions.
They refer to the carbon atoms on each monosaccharide that are connected by the glycosidic bond.
For example, “1–4” indicates a bond between carbon 1 of one sugar and carbon 4 of the next, which strongly influences the polymer’s geometry.
Yes. Some polysaccharides can be heteropolymers, built from more than one type of monosaccharide.
The sequence of monomers and the linkage types together determine the polymer’s chemical properties and how it interacts with water and proteins.
Practice Questions
Define the terms monosaccharide and polysaccharide. (2 marks)
Monosaccharide: a simple sugar / single carbohydrate monomer (1)
Polysaccharide: a complex carbohydrate polymer made of many monosaccharides bonded together (1)
Explain how monosaccharides can be joined to form a polysaccharide, and state two reasons why organisms build polysaccharides rather than keeping sugars only as monosaccharides. (6 marks)
Monosaccharides act as monomers that join to form larger carbohydrates (1)
Monosaccharides bond by forming covalent glycosidic linkages (1)
Linkage formation occurs via a condensation reaction producing water (1)
Any two of:
Polysaccharides allow energy to be stored in a compact form (1)
Polymers reduce osmotic effects compared with many free monosaccharides (1)
Polysaccharides can provide structural materials due to repeating linked units (1)

