OCR Specification focus:
‘Use a wide range of instruments and techniques appropriate to the specification and follow written instructions effectively.’
Using instruments and techniques correctly is essential for valid practical work. Competent biologists choose suitable tools, follow instructions methodically and apply practical skills consistently.
Following written instructions
Practical investigations must be carried out exactly as described to ensure repeatability, meaning the same person can obtain consistent results using the same method. Students must read the method thoroughly before beginning any task, identifying volumes, timings, temperatures and safety notes. Good practice includes highlighting hazards and planning the sequence of actions. Reproducibility, when different people or institutions achieve similar findings, also depends on clear methods and accurate execution. Careful adherence prevents procedural errors that can introduce bias or invalidate results.
Using a wide range of instruments and apparatus
OCR Biology requires familiarity with many laboratory tools. Students must understand their correct purpose, operation and limitations. Choosing inappropriate equipment can reduce precision and reliability.
Precision: The closeness of repeated measurements to each other, showing the level of random error present.
Common instruments include measuring cylinders, syringes, pipettes, water baths, thermometers, balances, microscopes, colorimeters, potometers and data loggers. Each instrument has an optimum use. For instance, a balance measures mass, while a colorimeter quantifies absorbance of light by a solution. Understanding resolution, the smallest measurable scale, is crucial when comparing tools, as higher resolution equipment improves the quality of data.
After selecting equipment, students must set it up safely and correctly. For electronic devices, calibration may be needed to ensure accurate baseline readings. Thermometers must be read at eye level, while meniscus readings in glassware must be taken at the lowest point of the curve. Some instruments require warm-up periods or blank samples before use, and accurate timing must accompany measurements.
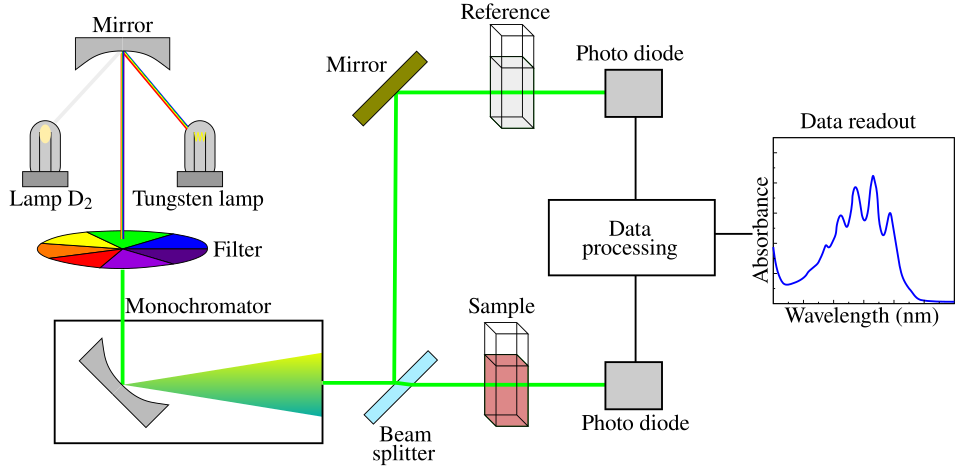
A schematic diagram showing the optical layout of a spectrophotometer, including the light source, sample position and detector. It illustrates where a blank and sample cuvette sit in the optical path, supporting correct zeroing and measurement technique. Source.
Key techniques in practical biology
Techniques must be executed consistently to prevent variation. Core techniques include:
• Heating using a water bath to maintain stable temperatures
• Preparing solutions by dissolving substances to known concentrations
• Producing serial dilutions to generate a range of concentrations
• Microscopy techniques such as focusing at low power before high power
• Measuring gas exchange or transpiration using potometers or sensors
• Using data software to present processed results graphically
Students should combine techniques smoothly and recognise sources of error. Recording instrument settings assists revisiting and refining methods.
Calibration and zeroing
Many instruments, such as balances and colorimeters, must be set to zero before use to eliminate background influence.

An analytical balance with a draft shield and digital readout, used for high-precision mass measurements. Closing the chamber and zeroing before weighing helps minimise error from air movement and residue. Source.
Calibration aligns the instrument with a known standard so that future readings are accurate. Balanced calibration weights or blank cuvettes may be used. Failure to calibrate introduces systematic error, shifting all results in one direction.
Systematic error: A consistent deviation from the true value due to a flaw in equipment or method.
After calibration, readings must still be taken with care. For example, when using a microscope, stage micrometers and eyepiece graticules allow size measurements to be standardised between sessions.
Accurate timing, temperature control and measurement
Many biological reactions are temperature-dependent, so thermostatic control is essential. Water baths and thermostatically controlled incubators maintain suitable conditions. Timers ensure equal reaction periods, and measurements should be logged immediately to avoid memory-based inaccuracies. Good practical technique includes repeating measurements and using appropriate decimal places based on the instrument scale.
Using software tools with instruments
Modern practical work often links instruments with software that records values, draws graphs or performs statistics. Data loggers can measure light, humidity or pH continuously, reducing human error. Graphing software helps visualise trends and identify anomalies. Students must label axes, include units and select suitable scales to communicate findings clearly.
Checklists for effective technique
To maximise success, students should:
• Read all instructions before starting, noting hazards and controls
• Select instruments with appropriate resolution and range
• Calibrate equipment where necessary and zero before recording
• Keep conditions such as temperature and time constant
• Record all data clearly in tables with units
• Follow aseptic and safety procedures when required
• Repeat measurements to improve reliability
Following written instructions and using instruments skilfully supports valid outcomes and reflects best scientific practice.
FAQ
Choose the instrument that can measure to the smallest useful scale while still matching the range of data you expect to collect. A balance that measures to 0.001 g offers finer resolution than one that measures to 0.1 g, but it is only beneficial if the sample masses are small enough for this to matter.
Check the instrument’s scale or digital display, and match its precision to the level of detail needed in your investigation. Avoid choosing equipment that provides unnecessary resolution, as this can increase time and complexity without improving data quality.
Accuracy describes how close a measurement is to the true value, while precision refers to how close repeated readings are to each other.
Sensitivity refers to the smallest detectable change an instrument can measure. A more sensitive instrument can show changes that a less sensitive one might miss.
High precision does not guarantee accuracy, especially if systematic error is present, so calibration remains essential.
Environmental changes can alter sensor readings and create variation that is unrelated to the variable being tested.
For example, light sensors, humidity probes or temperature loggers may respond to small fluctuations in the surroundings. To minimise this:
• Use insulation or shielding
• Allow devices to stabilise before recording data
• Repeat measurements under the same conditions
Consistency ensures that patterns in the data reflect real biological changes rather than random environmental influence.
First repeat the measurement to check for random error. If the issue persists, recalibrate or zero the instrument and ensure it is set up correctly.
Examine possible mechanical or electronic faults, such as air bubbles in colorimeter cuvettes or loose connections on sensors. Switch to a different instrument if problems continue so results are not compromised.
Good practice includes recording any irregularities and explaining how they were addressed in the method or evaluation.
Clear instructions should include precise volumes, timings, temperatures, equipment names and safety notes.
To increase clarity:
• Use numbered steps in chronological order
• Specify resolution or model of instruments if relevant
• State how many repeats are required
• Indicate when calibration or zeroing is needed
Well-designed instructions reduce ambiguity, making it more likely that different people will carry out the method in the same way and obtain comparable results.
Practice Questions
Question 1 (2 marks)
State two reasons why scientific instruments, such as balances or colorimeters, must be zeroed or calibrated before taking measurements in practical investigations.
Question 1 (2 marks)
Award one mark for each valid point, up to two marks:
• To remove baseline or background readings and ensure measurements start from zero (1)
• To reduce systematic error and improve accuracy (1)
• To ensure readings are directly comparable and standardised (1)
(Any two)
Question 2 (5 marks)
A student is using a colorimeter to investigate the effect of enzyme concentration on the breakdown of a substrate. The results from different repeats are highly variable. Describe the steps the student should take, when using instruments and following written instructions, to improve the reliability and accuracy of the data collected.
Question 2 (5 marks)
Award up to five marks for the following points:
• Calibrate or zero the colorimeter using an appropriate blank before each set of readings (1)
• Follow the method step-by-step without altering timings, volumes or temperatures (1)
• Ensure cuvettes are clean, handled by the frosted sides and placed in the same orientation each time to avoid interference (1)
• Keep all other variables constant by controlling temperature, using a water bath or thermostat where appropriate (1)
• Take multiple repeats and calculate a mean to improve reliability (1)
• Record measurements immediately and with appropriate precision for the instrument (1)
(Any five)

