OCR Specification focus:
‘Explain monomers, polymers, condensation and hydrolysis in forming and breaking glycosidic bonds.
Carbohydrates are essential biological molecules built from sugar monomers that bond to form polymers; their structures and reactions underpin key cellular functions and energy production.
Carbohydrates: monomers, polymers and glycosidic bonds
Carbohydrates are a major group of biological molecules made of carbon, hydrogen and oxygen, typically in the ratio CH₂O. They include simple sugars and complex macromolecules and play structural and metabolic roles in cells.
Monomers and monosaccharides
The basic molecular units of carbohydrates are monosaccharides, which are single sugar molecules such as glucose, galactose and fructose. These monomers dissolve easily in water and are an immediate source of energy for respiration.
Monomer: A small, basic molecular unit that can join with others to form a larger polymer.
Monosaccharides contain a carbon skeleton with a carbonyl group and multiple hydroxyl groups, allowing them to react to form larger carbohydrates. They can exist in linear or ring forms, with ring structures being dominant in aqueous environments. Glucose is especially important in biology and exists as α-glucose and β-glucose, which differ in the position of the hydroxyl group on carbon 1.
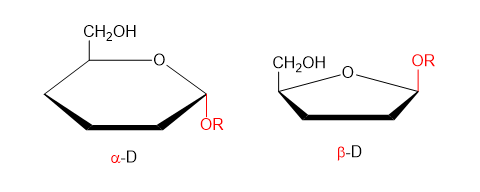
Haworth projections of α- and β-glucose showing the different orientation of the hydroxyl group on carbon 1, which underlies α and β glycosidic bond formation in carbohydrates. Source.
Carbohydrate polymers
When many monosaccharide monomers join together, they form long-chain molecules called polysaccharides such as starch, glycogen and cellulose.
Polymer: A large molecule made from many repeating monomer units bonded together.
Carbohydrate polymers may be branched or unbranched and may form compact spirals or straight, rigid fibres. Their properties depend on the monomer used, the type of glycosidic bonds present and the degree of branching. For example, α-glucose forms spiralled or branched polymers suited for energy storage, whereas β-glucose produces straight chains that are ideal for structural support in plant cell walls.
Condensation reactions and the formation of glycosidic bonds
Carbohydrate monomers join through condensation reactions, which involve the removal of a molecule of water. This reaction produces a glycosidic bond, the covalent linkage between two sugar residues.
Glycosidic bond: A covalent bond formed between two monosaccharides during a condensation reaction, releasing water.
A disaccharide is formed when two monosaccharides condense.
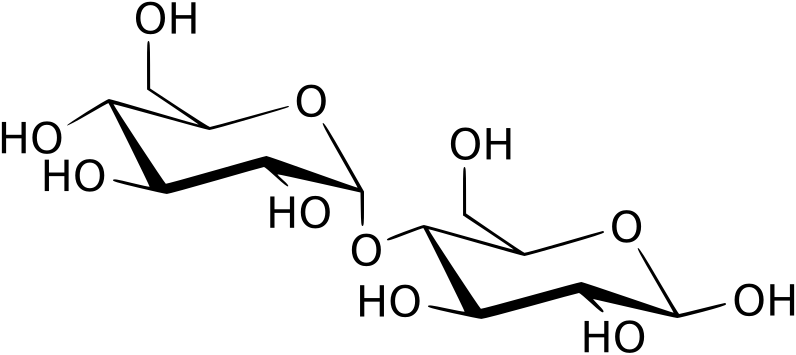
Structure of maltose showing an α(1→4) glycosidic bond between two glucose monomers formed by a condensation reaction that releases water. Source.
Common biological examples include:
Maltose (glucose + glucose)
Sucrose (glucose + fructose)
Lactose (glucose + galactose)
To form a glycosidic bond, the hydroxyl groups on carbon 1 of one monosaccharide and carbon 4 of another interact. This creates a 1,4-glycosidic bond, although other link positions such as 1,6-glycosidic bonds can also form, producing branching in polymers.
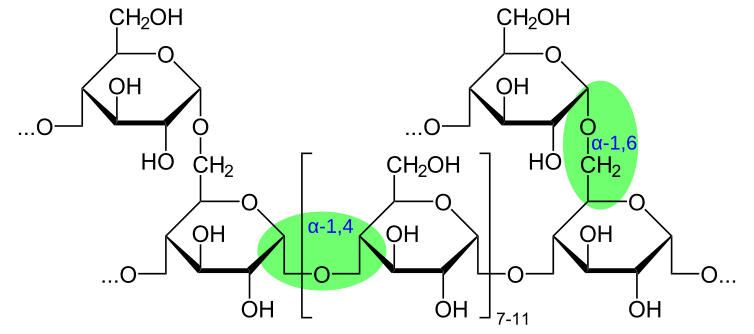
Diagram of glycogen demonstrating α-1,4 glycosidic bonds along chains and α-1,6 bonds at branch points, which create a compact, branched polysaccharide structure. Source.
Condensation is central to building complex carbohydrates:
Monosaccharides → (condensation) → disaccharides
Disaccharides → (condensation) → polysaccharides
Hydrolysis and carbohydrate breakdown
The reverse of condensation is hydrolysis, a reaction in which water is added to break glycosidic bonds. Hydrolysis reactions are vital in digestion, respiration and cellular metabolism.
Hydrolysis: The breakdown of a bond within a molecule using water.
Hydrolysis reactions are catalysed by specific enzymes, ensuring that polysaccharides can be rapidly converted back into soluble monosaccharides when energy is needed. For example, starch is hydrolysed to maltose by amylase, and maltose is then hydrolysed to glucose by maltase.
Bond types and structural implications
The orientation of monomers determines whether the glycosidic bond is α or β, and this has major biological consequences. α-glycosidic bonds in starch and glycogen allow coils and branches that are easily hydrolysed, enabling rapid energy release. In contrast, β-glycosidic bonds in cellulose cause each glucose to rotate, producing straight chains that hydrogen-bond into strong microfibrils.
Key features of condensation and hydrolysis in carbohydrates
Condensation forms glycosidic bonds and releases water
Hydrolysis breaks glycosidic bonds using water
Enzymes provide specificity for both processes
Bond position (1,4 or 1,6) controls structure and function
α- and β-isomers lead to storage versus structural roles
Biological relevance
Carbohydrate monomers and polymers are essential for energy transfer, energy storage and structural integrity. Glucose fuels ATP production in respiration, while large polymers such as starch, glycogen and cellulose support survival in different organisms. Understanding how condensation and hydrolysis build and break glycosidic bonds is therefore fundamental in explaining carbohydrate function in living systems.
FAQ
Glycosidic bonds are covalent bonds, which makes them chemically stable under normal conditions. Their stability comes from shared electrons between the bonded monosaccharides, creating a strong link.
Despite this, the body breaks them efficiently because enzymes lower the activation energy. Enzymes position reactants correctly and strain the bond, allowing hydrolysis to occur rapidly at body temperature.
The 1,4 arrangement allows monosaccharides to form long, unbranched or gently coiled chains that are structurally stable and compact.
This produces polysaccharides that are efficient for storage or strength, depending on the monomer. For example:
Alpha 1,4 bonds form coiled energy storage chains
Beta 1,4 bonds form straight, rigid structural chains
The difference lies in the orientation of the hydroxyl group on carbon 1. This small structural change alters bond angles and how monomers align during condensation.
As a result:
α-glucose forms spiralled or branched chains
β-glucose forms straight, cross-linked chains
This leads to different properties and biological roles.
Each enzyme has an active site with a precise shape and chemical environment. Only substrates forming a specific glycosidic bond can bind correctly to the active site.
For example, an enzyme that hydrolyses α(1→4) bonds will not recognise β(1→4) bonds because the orientation of groups in the substrate is different, preventing a successful enzyme–substrate complex.
1,6 bonds introduce branch points, allowing more chain ends to form. Hydrolytic enzymes can access many ends at once, greatly speeding up glucose release.
Branching also makes the molecule compact and more soluble, which is important for rapid mobilisation of stored energy in cells.
Practice Questions
Question 1 (2 marks)
Monosaccharides can join together to form disaccharides. Name the type of reaction involved in this process and identify the bond formed between the two monosaccharides.
Question 1 (2 marks)
Condensation reaction (1 mark)
Glycosidic bond (1 mark)
Question 2 (5 marks)
Describe how glycosidic bonds are formed and broken in carbohydrates. In your answer, refer to the role of water, the names of the reactions involved, and how enzymes contribute to these processes.
Question 2 (5 marks)
Award up to five marks for the following points:
Glycosidic bonds are formed through a condensation reaction (1 mark)
Water is released during bond formation (1 mark)
Glycosidic bonds are broken by hydrolysis (1 mark)
Hydrolysis uses water to break the bond (1 mark)
Enzymes (e.g., carbohydrases) catalyse the reactions, ensuring they occur at a suitable rate (1 mark)

