OCR Specification focus:
‘Relate hydrogen bonding to water’s properties: solvent, transport medium, coolant and habitat.’
Water is fundamental for life because its molecular structure and extensive hydrogen bonding give rise to unusual properties that support biochemical reactions, transport, temperature regulation and survival in diverse environments.
Structure of Water and Hydrogen Bonding
Water is a polar molecule formed when oxygen covalently bonds to two hydrogen atoms, producing an uneven distribution of charge. Oxygen, being more electronegative, attracts electrons more strongly, creating a slight δ− charge on the oxygen atom and δ+ charges on the hydrogens. The polarity enables water molecules to form hydrogen bonds with each other and with other polar substances.
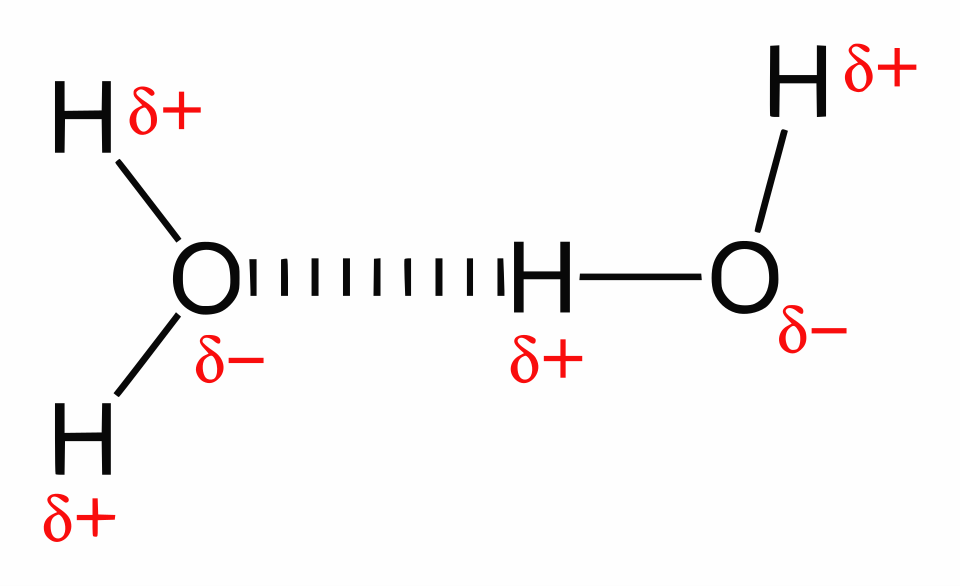
A labelled 2D diagram illustrating hydrogen bonding between water molecules, highlighting polarity and the attraction between δ+ hydrogens and δ− oxygens that drives hydrogen bond formation. Source.
Hydrogen bond: A weak electrostatic attraction between a slightly positive hydrogen atom and a slightly negative atom (often oxygen or nitrogen) on a neighbouring molecule.
Hydrogen bonds are individually weak, but collectively strong because each water molecule can form up to four hydrogen bonds. This creates a cohesive, lattice-like arrangement in liquid water and an even more open structure in ice. Although hydrogen bonds constantly break and reform, they require significant energy to overcome, affecting water’s thermal stability and surface behaviour.
Water as a Solvent
Water is a highly effective solvent for ionic and polar molecules, allowing metabolic chemistry to occur in solution. Because opposite charges attract, positive ions (cations) are surrounded by δ− oxygen regions, while negative ions (anions) interact with δ+ hydrogen regions. This process, known as dissociation, enables dissolved substances to move freely and react.
Water’s solvent properties support essential biological roles:
Medium for metabolic reactions, including enzyme activity and transport of substrates and products.
Transport of ions and solutes in blood, phloem sap and xylem.
Efficient chemical interaction, as dissolved solutes diffuse and collide more easily.
Water as a Transport Medium
Hydrogen bonding gives water cohesion (attraction between water molecules) and adhesion (attraction between water and other surfaces). These properties allow water to move in continuous columns, vital in biological transport systems.
Cohesion: Attraction between molecules of the same substance.
Because of cohesion, water generates high surface tension and supports capillary action, allowing upward flow against gravity. In xylem vessels, cohesive forces maintain unbroken water columns, enabling transpiration pull from roots to leaves.
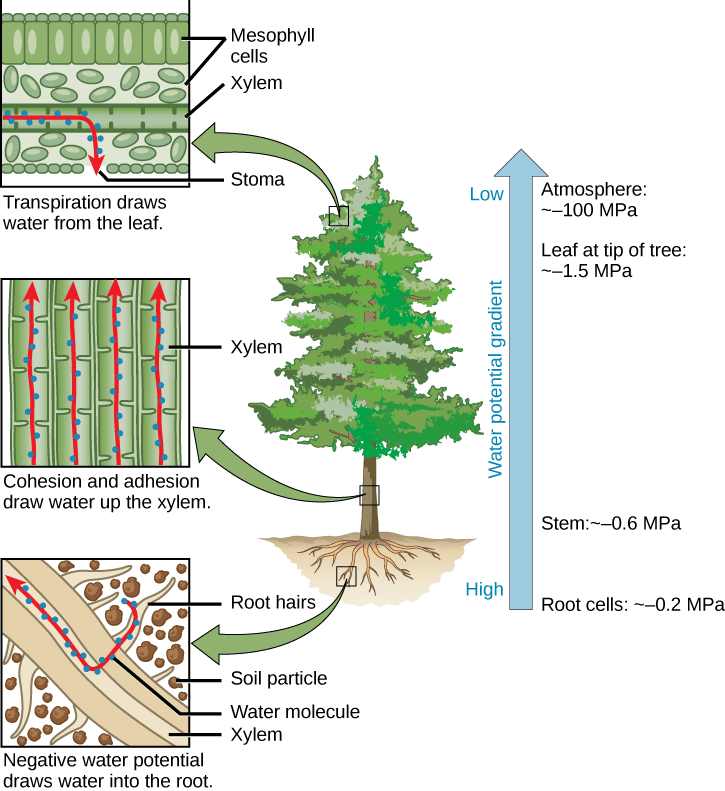
A diagram of the cohesion–tension mechanism in xylem, showing how evaporation at leaves generates tension and cohesion pulls continuous water columns upward through the plant. Source.
In animals, water’s incompressibility allows it to act as an effective transport medium in blood, carrying hormones, glucose, amino acids and gases.
Water as a Coolant
Large amounts of energy are required to break hydrogen bonds, leading to high specific heat capacity and high latent heat of vaporisation. These thermal properties allow water to resist rapid temperature change and absorb heat during evaporation.
Specific heat capacity: The energy required to raise the temperature of 1 kg of a substance by 1 °C.
As a coolant, water:
Maintains stable internal conditions, protecting enzymes from denaturation.
Supports homeostasis by limiting temperature fluctuations in organisms and habitats.
Cools surfaces by evaporation, e.g., sweating in mammals or transpiration from leaves, as energetic molecules escape as vapour, removing heat.
Water as a Habitat
Hydrogen bonding creates a stable aquatic environment. Water has a high density, especially in liquid form, supporting buoyancy and preventing rapid temperature shifts that could threaten life. Importantly, ice is less dense than liquid water because hydrogen bonds hold molecules in an open lattice. Ice floats, forming an insulating layer on ponds and lakes, keeping water beneath liquid and allowing organisms to survive through winter.
As a habitat, water offers:
Support and buoyancy for aquatic organisms.
Thermal stability, as large bodies of water warm and cool slowly.
Light penetration, enabling photosynthesis in aquatic plants and algae.
Molecular Interactions and Additional Properties
Water’s hydrogen bonding explains further biologically important characteristics:
High surface tension, allowing small organisms such as pond skaters to move across the surface.
Transparency, enabling underwater photosynthesis.
Incompressibility, allowing it to function in hydraulic mechanisms, such as plant support through turgor pressure.
Turgor pressure: The pressure exerted by the cell contents against the cell wall when water enters by osmosis.
Together, these properties ensure water acts simultaneously as a solvent, transport medium, coolant and habitat, exactly as required by the OCR specification.
FAQ
Hydrogen bonds are weak intermolecular forces, so the kinetic energy of water molecules causes them to break frequently. However, because there are many potential bonding sites, new hydrogen bonds rapidly reform with neighbouring molecules.
This balance gives water fluidity while still maintaining overall cohesion between molecules.
Non-polar molecules, such as many lipids, cannot form favourable interactions with water and are excluded from aqueous environments. This drives hydrophobic interactions that help form structures like phospholipid bilayers.
As a result, non-polar substances tend to cluster together, reducing their contact with water.
When water cools, hydrogen bonds fix molecules into a rigid lattice with more open space, lowering its density. This causes ice to float on liquid water.
Floating ice forms an insulating layer, preventing complete freezing below and allowing aquatic organisms to survive through winter.
Cohesion between water molecules creates a 'skin' at the surface due to strong hydrogen bonding. This surface tension resists external force.
Small organisms, such as pond skaters, distribute their mass over a large enough area to avoid breaking the hydrogen-bonded surface layer.
Because water resists rapid temperature change, cells maintain stable internal conditions, protecting enzymes from denaturation.
This thermal buffering keeps metabolic reactions operating at a consistent rate, even when external temperatures fluctuate.
Practice Questions
Question 1 (2 marks)
Explain how hydrogen bonding in water leads to one named biological property.
Question 1 (2 marks)
1 mark for hydrogen bonding statement:
Hydrogen bonds form between the slightly positive hydrogen of one water molecule and the slightly negative oxygen of another.
1 mark for link to property:This causes cohesion / high specific heat capacity / high latent heat of vaporisation / water to remain liquid over a wide range of temperatures (any one suitable property, correctly linked).
Question 2 (5 marks)
Water plays several key roles in organisms. Describe how the structure of water enables it to act as:
• a solvent
• a transport medium
• a coolant
Question 2 (5 marks)
Up to 5 marks, one mark per distinct point:
Solvent (max 2 marks)
Water is a polar molecule.
It surrounds and dissolves ionic or polar substances by forming hydration shells.
Transport medium (max 2 marks)
Cohesion allows water molecules to stick together, forming continuous columns.
This enables movement in xylem or blood as water is incompressible and flows easily.
Coolant (max 2 marks)
High latent heat of vaporisation means evaporation removes large amounts of heat.
High specific heat capacity buffers temperature change to maintain stable internal conditions.
(Any 5 valid points, clearly linked to the roles stated.)

