OCR Specification focus:
‘Compare α- and β-glucose; describe sucrose, lactose, maltose; outline starch, glycogen and cellulose.’
Glucose, disaccharides and polysaccharides are central to biological chemistry, providing vital energy and structural roles; their bonding, isomerism and polymer formation underpin key cellular processes in living organisms.
Structure of Glucose
Glucose is a hexose monosaccharide that acts as a primary respiratory substrate in cells. Monosaccharides are simple sugars that consist of a single monomer unit and form larger carbohydrates through condensation reactions.
Monosaccharide: A single sugar molecule that cannot be hydrolysed into smaller carbohydrate units.
Glucose has two structural forms: α-glucose and β-glucose, which are structural isomers. Structural isomers have the same molecular formula (C₆H₁₂O₆) but different arrangements of atoms. In α-glucose, the hydroxyl (–OH) group on carbon 1 is below the ring, whereas in β-glucose it is above.
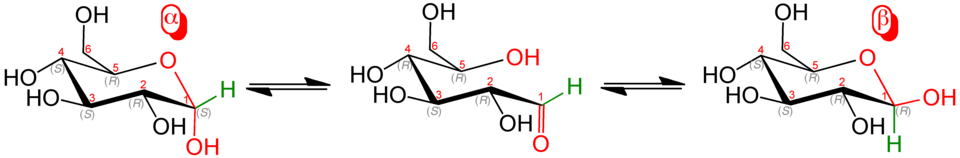
The α-anomer shows the C1 hydroxyl positioned downward, while the β-anomer shows it pointing upward, clearly illustrating the structural difference that leads to major functional contrasts in polysaccharides. Source.
Formation of Disaccharides
Disaccharides are formed when two monosaccharides join by a condensation reaction, creating a glycosidic bond and releasing water.
Glycosidic bond: A covalent bond formed between two monosaccharides during a condensation reaction.
Disaccharides can be broken down by hydrolysis, which requires water and is catalysed by specific enzymes. Different pairs of monosaccharides form distinct biologically important disaccharides used in transport or as energy sources.
Key Disaccharides
Maltose: formed from α-glucose + α-glucose; important in digestion of starch.
Sucrose: formed from α-glucose + fructose; major transport sugar in plants.
Lactose: formed from β-galactose + glucose; found in mammalian milk.
Each disaccharide has a specific 1,4-glycosidic bond, although other positions can occur in nature. These molecules are soluble and suitable for transport and short-term energy storage.
Biological Polysaccharides
Polysaccharides are polymers of monosaccharides, formed by repeated condensation reactions producing long chains. Their properties vary depending on the monomer, glycosidic bond type and degree of branching. They are typically insoluble, making them suitable for storage or structural roles.
Polymer: A large molecule made of repeated monomer units chemically bonded in a chain.
There must be a clear biological advantage to their insolubility and compact structure, especially in cells needing efficient storage or strong structural materials.
Starch — plant storage polysaccharide
Starch is a plant storage molecule made of α-glucose and consists of two components:
Amylose: long, unbranched chains with 1,4-glycosidic bonds forming a spiral held by hydrogen bonds. This structure is compact and ideal for storage.
Amylopectin: branched chains with 1,4- and 1,6-glycosidic bonds, enabling rapid hydrolysis by enzymes and quick glucose release.
Key properties of starch:
Insoluble, so does not affect osmotic potential in cells
Compact, maximising storage capacity
Readily hydrolysed, providing a reliable energy source
Glycogen — animal and fungal storage polysaccharide
Glycogen is the main storage carbohydrate in animals and fungi, also composed of α-glucose, but it is more highly branched than amylopectin. This structure:
Increases the number of free ends for rapid enzyme action
Allows swift release of glucose to meet high metabolic demands
Its compact and insoluble nature makes glycogen well suited for storage in liver and muscle tissues.
Cellulose — plant structural polysaccharide
Cellulose is a structural polysaccharide made of β-glucose. Each β-glucose rotates 180° before bonding, forming long straight chains with 1,4-glycosidic bonds. These chains run parallel and form strong hydrogen bonds, grouping into microfibrils that provide high tensile strength.
This structure is essential for the plant cell wall, giving rigidity, support and resistance to osmotic lysis. The β-glucose orientation prevents coiling, contrasting sharply with α-glucose polysaccharides.
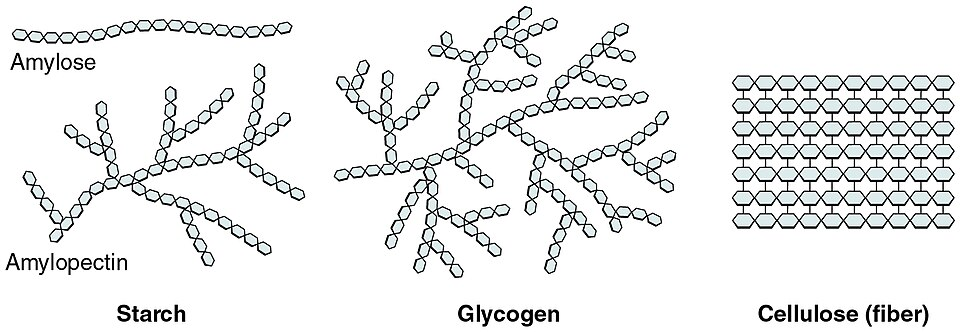
The image shows amylose as an unbranched α-1,4 chain, amylopectin as a branched α-1,4/α-1,6 chain, glycogen as a highly branched α-glucan, and cellulose as a β-1,4 straight chain, visually reinforcing how linkage type dictates function. Source.
Summary of Structural Links
α-glucose → starch and glycogen used for energy storage
β-glucose → cellulose used for structural support
Disaccharides act as transport sugars and intermediates in metabolism
Key Features Across Carbohydrates
Condensation creates glycosidic bonds and water
Hydrolysis breaks polysaccharides and disaccharides into monosaccharides
Structure and bonding determine biological function
Solubility and branching influence storage efficiency and accessibility
FAQ
Alpha- and beta-glucose form different polysaccharides because their anomeric hydroxyl groups face in opposite directions. Enzymes involved in carbohydrate synthesis and digestion are highly specific, so they only recognise one anomer.
As a result:
Alpha-glucose forms starch and glycogen, which coil or branch and act as storage molecules.
Beta-glucose forms cellulose, which builds into straight, strong fibres for structure.
This structural variation underpins key biological roles in plants and animals.
The digestive enzyme amylase hydrolyses alpha-1,4-glycosidic bonds in starch, so humans can break it down.
However, cellulose contains beta-1,4-glycosidic bonds that require cellulase, an enzyme humans do not produce. Cellulose chains also form tightly packed microfibrils, which further limit enzyme access.
This is why cellulose functions as dietary fibre in humans rather than an energy source.
Branching creates many terminal glucose residues, so enzymes can hydrolyse multiple points at once.
This leads to:
Faster glucose release for respiration.
Compact storage because branched chains pack efficiently
Greater solubility compared to long, unbranched chains.
Highly branched glycogen therefore supports rapid metabolic demand in animals, particularly in muscle and liver cells.
Sucrose is a non-reducing sugar, so it is chemically stable during long-distance transport in the phloem.
It is also:
Less reactive than glucose, preventing damage to transport tissues.
Soluble for easy movement through phloem sap.
Metabolically versatile, as it can be hydrolysed into glucose and fructose at the destination.
These properties make sucrose ideal for translocation in plants.
Every beta-glucose rotates 180 degrees before bonding, producing straight chains that lie parallel to each other.
Hydrogen bonds form between adjacent chains, grouping them into microfibrils. These microfibrils:
Provide tensile strength
Resist osmotic swelling
Form a rigid network within the plant cell wall
The cumulative strength of many hydrogen bonds gives cellulose exceptional stability.
Practice Questions
Question 1 (1–3 marks)
Explain how the structure of cellulose differs from the structure of starch.
Question 1 Mark Scheme (max 3 marks)
Award 1 mark for each relevant point:
Cellulose is formed from beta-glucose, whereas starch is formed from alpha-glucose. (1)
Cellulose has 1,4-glycosidic bonds forming straight, unbranched chains; starch contains amylose (1,4 bonds) and amylopectin (1,4 and 1,6 bonds). (1)
Cellulose chains form hydrogen bonds between molecules to produce strong microfibrils, whereas starch chains coil or branch and do not form microfibrils. (1)
Question 2 (4–6 marks)
Describe how disaccharides and polysaccharides are formed from monosaccharides, and explain how the bonding patterns in starch and glycogen relate to their roles as storage molecules.
Question 2 Mark Scheme (max 6 marks)
Award 1 mark for each relevant point:
Disaccharides and polysaccharides form through condensation reactions between monosaccharides. (1)
A glycosidic bond is created and water is released during condensation. (1)
Hydrolysis reactions break glycosidic bonds using water. (1)
Starch and glycogen both contain alpha-glucose. (1)
Amylose and amylopectin in starch, and glycogen in animals, contain 1,4-glycosidic bonds, while amylopectin and glycogen also contain 1,6-glycosidic bonds for branching. (1)
Branching provides many terminal glucose units, allowing rapid hydrolysis and release of glucose for respiration, making starch and glycogen effective storage molecules. (1)

