OCR Specification focus:
‘Describe globular and fibrous proteins, key inorganic ions, biochemical tests, colorimetry and chromatography with Rf.’
Proteins with contrasting structures enable diverse biological functions, while inorganic ions support essential physiological roles. Accurate identification of functional molecules requires biochemical tests, alongside quantitative and separation techniques used to analyse mixtures.
Globular and Fibrous Proteins
Globular proteins are compact, roughly spherical proteins that are soluble in water due to hydrophilic residues orientated outwards.
Fibrous proteins are long, insoluble molecules with repetitive amino acid sequences forming strong structural fibres.
Globular protein: A compact, soluble protein with metabolic or functional roles such as catalysis, transport or regulation.
Examples of globular proteins include haemoglobin, insulin and catalase, each with a precise tertiary or quaternary structure enabling substrate binding or molecular transport.
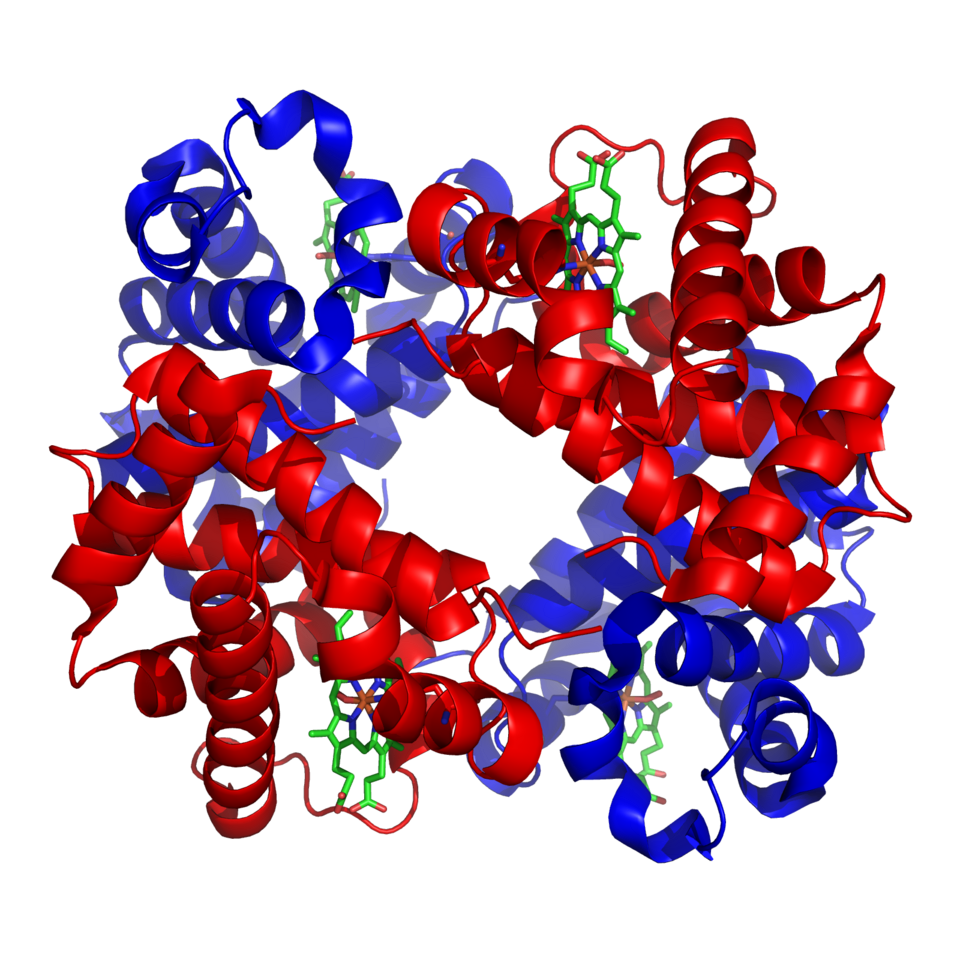
Ribbon diagram of haemoglobin showing its compact, folded polypeptide chains, demonstrating a typical globular protein with quaternary structure and outward-facing hydrophilic groups. Source.
Their complex folding is stabilised by hydrogen bonds, ionic interactions, hydrophobic interactions and disulphide bridges. Hydrophilic R groups face outwards, aiding solubility in aqueous environments such as blood and cytoplasm.
In contrast, fibrous proteins such as collagen, keratin and elastin are structurally supportive.
Triple-helical collagen showing three polypeptide chains wound into a rope-like structure, illustrating tensile strength and insolubility characteristic of fibrous proteins. Source.
They are formed from polypeptide chains arranged into fibres that provide high tensile strength, flexibility or protective rigidity. Their insolubility arises from hydrophobic R groups on the exterior and extensive cross-linking.
Fibrous protein: An elongated, insoluble protein with structural roles based on repetitive amino acid sequences forming strong fibres.
Key Inorganic Ions
Biological systems rely on inorganic ions that support enzyme activity, transport and structural stability. Many act as cofactors, enabling enzymes to catalyse reactions efficiently. Important ions include:
Calcium (Ca²⁺): strengthens bones and teeth; stabilises cell walls and membranes; regulates neurotransmission and muscle contraction.
Sodium (Na⁺) and Potassium (K⁺): central to nerve impulse transmission and maintaining membrane potential through the sodium–potassium pump.
Hydrogen (H⁺): determines pH levels and influences enzyme activity by affecting protein structure.
Nitrate (NO₃⁻): required for amino acid and nucleic acid synthesis in plants.
Chloride (Cl⁻): acts as a cofactor for amylase and helps maintain electrical neutrality in cells.
Phosphate (PO₄³⁻): forms part of ATP, phospholipids and nucleotides, essential in energy transfer and membrane structure.
A normal sentence must follow before moving to biochemical testing techniques, which enable these molecules to be identified and analysed in laboratory settings.
Biochemical Tests for Functional Molecules
OCR requires knowledge of core qualitative food tests. Students must understand the need for positive and negative controls. Key tests include:
Benedict’s reagent for reducing sugars, producing a brick-red precipitate when heated with reducing sugars such as glucose.
Iodine solution for starch, turning blue-black in its presence.
Biuret test for proteins, resulting in a lilac colour when peptide bonds are present.
Ethanol emulsion test for lipids, forming a white emulsion when lipids disperse in water after dissolving in ethanol.
These colour changes allow identification of specific macromolecules. However, qualitative tests lack precision, so quantitative methods are also employed.
Colorimetry
Colorimetry measures the absorbance or transmission of light through a coloured solution to estimate concentration. In food testing this is commonly used with Benedict’s test for reducing sugar.
Students must understand the following process:
Prepare known standard concentrations to generate a calibration curve.
Use a colorimeter with an appropriate filter to measure absorbance.
Plot concentration against absorbance.
Use the graph to determine the concentration of an unknown sample.
EQUATION
—-----------------------------------------------------------------
Calibration (Beer–Lambert relationship) (A = kC)
A = Absorbance (no unit)
C = Concentration (mol dm⁻³)
k = Constant of proportionality (depends on path length and substance)
—-----------------------------------------------------------------
A normal sentence must appear here to separate equations from chromatographic analysis, which allows mixtures to be separated based on molecular properties.
Chromatography and Rf Values
Chromatography is a separation technique where molecules partition between a stationary phase and a mobile phase. More soluble molecules travel further with the mobile phase, enabling separation of amino acids or pigments.
EQUATION
—-----------------------------------------------------------------
Rf Value (Rf) = Distance moved by substance ÷ Distance moved by solvent front
Rf = Relative solubility (no unit)
—-----------------------------------------------------------------
Key chromatographic points for OCR:
Baseline drawn in pencil to avoid ink contamination.
Spots applied sparingly to prevent overlap.
Solvent below the origin to stop dissolving of samples into the reservoir.
Separation reflects differences in solubility and interactions such as hydrogen bonding.
Chromatography enables identification by comparing Rf values with known standards, supporting qualitative analysis of biological mixtures.
FAQ
Globular proteins fold so that hydrophilic R groups face outward, forming hydrogen bonds with surrounding water, which increases solubility. Hydrophobic groups are buried inside the molecule, stabilising the tertiary structure.
Fibrous proteins, however, have long, repetitive sequences with many hydrophobic R groups on their surface. These form cross-links between molecules, creating strong, insoluble fibres rather than soluble structures.
Collagen consists of three polypeptide chains tightly wound into a triple helix. Every third amino acid is glycine, allowing the chains to pack closely.
Multiple helices are further stabilised by covalent cross-links between adjacent molecules. This layered, rope-like arrangement resists stretching, giving collagen its high tensile strength.
A colorimeter only measures light absorbance or transmission; it does not directly give concentration. A calibration curve links absorbance to known sample concentrations.
Once plotted, the curve allows students to determine the concentration of an unknown sample by reading across from its absorbance value. This improves accuracy and reduces reliance on subjective colour comparisons.
Several experimental variables can alter solvent movement or molecular behaviour, including:
Use of ink instead of pencil, causing baseline contamination
Overloaded or uneven sample spots
Temperature fluctuations
Solvent evaporation during the run
Small errors in measuring distances after the solvent front has run also lead to incorrect Rf calculations.
If the solvent covers the baseline, the samples dissolve directly into the solvent reservoir. This prevents separation because molecules do not travel with the mobile phase up the chromatography medium.
By keeping the baseline above the solvent level, the samples are carried upwards as the solvent front moves, allowing different solubilities to produce distinct, separable spots.
Practice Questions
Question 1 (2 marks)
Name one globular protein and one fibrous protein, and state one function for each.
Question 1 (2 marks)
Award 1 mark for a correct named example of each protein type, and 1 mark for a correct function.
Accept the following, or other suitable examples:
Globular protein: haemoglobin, catalase or insulin (1 mark)
Function: oxygen transport / catalysis / blood glucose regulation (must match the named protein) (1 mark)Fibrous protein: collagen, keratin or elastin (1 mark)
Function: structural support / waterproofing / elasticity (must match the named protein) (1 mark)
Maximum 2 marks.
Question 2 (5 marks)
A student conducts chromatography on a mixture of amino acids. Explain how chromatography separates the amino acids, and how the student could identify which amino acids are present in the mixture. In your answer, refer to both the process and the use of Rf values.
Question 2 (5 marks)
Award marks for the following valid points, up to 5 marks total:
Molecules separate based on differences in solubility or affinity between the stationary phase and mobile phase (1 mark)
More soluble amino acids travel further up the chromatography medium (1 mark)
A baseline must be drawn in pencil and spots applied above the solvent line (1 mark)
Distance moved by each spot is measured and Rf values are calculated (1 mark)
Identification is made by comparing calculated Rf values with known standards (1 mark)
Maximum 5 marks.

