OCR Specification focus:
‘Describe saturated and unsaturated fatty acids; form and hydrolyse ester bonds; relate lipid structure to function.’
Lipids are diverse biological molecules important for membranes, energy storage and signalling. Triglycerides, phospholipids and cholesterol have distinct structures giving specialised properties in cells and organisms.
Triglycerides
Triglycerides are the main energy storage lipids in animals and plants. They are formed from fatty acids and glycerol, a three-carbon alcohol with three hydroxyl (–OH) groups that enable bond formation.
Fatty acid: A hydrocarbon chain with a carboxyl (–COOH) group that is either saturated or unsaturated.
Saturated fatty acids contain only single C–C bonds, forming straight hydrocarbon chains that pack closely together, increasing the melting point and producing solid fats. Unsaturated fatty acids contain one or more C=C double bonds, introducing kinks that prevent tight packing, lowering the melting point and producing oils.
Ester bond: A covalent bond formed between a fatty acid and glycerol in a condensation reaction, releasing water.
Triglycerides are synthesised by condensation reactions, where three fatty acids bond to glycerol via esterification. This produces one triglyceride and three molecules of water.
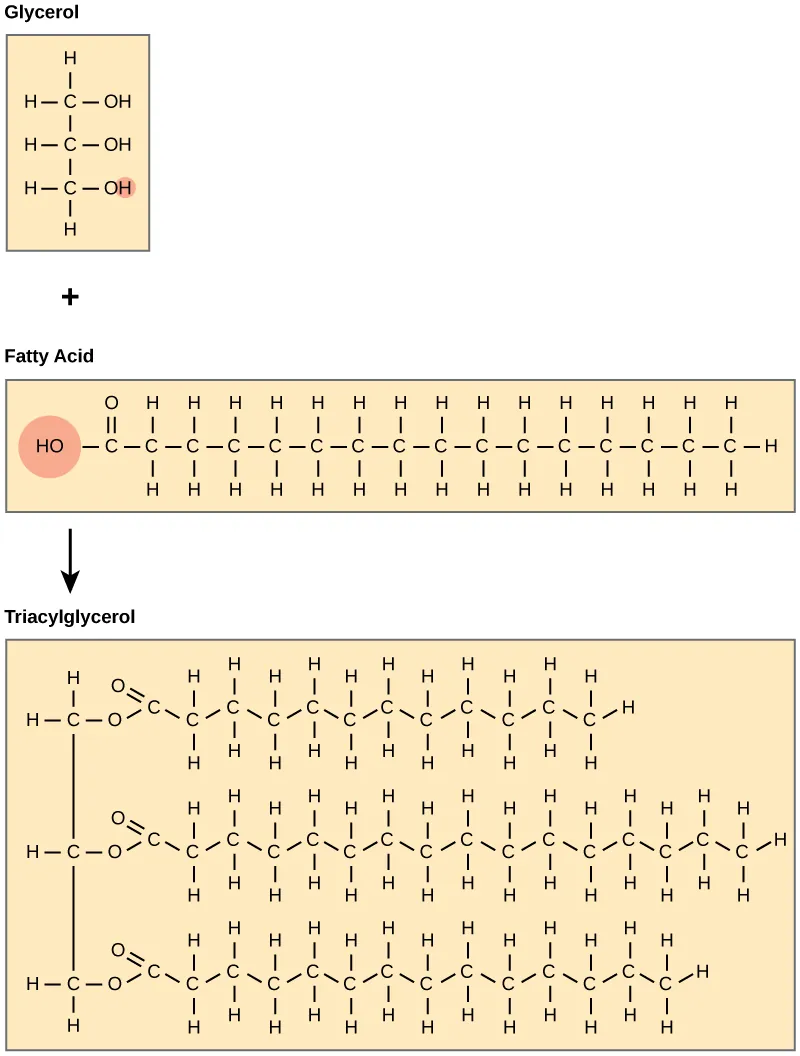
Dehydration (condensation) links three fatty acids to glycerol via ester bonds, forming a triglyceride and releasing three water molecules. This diagram also labels glycerol, fatty acids and the ester linkages, matching OCR-required terminology. Source.
They are broken down by hydrolysis reactions, which require water to split ester bonds and release the fatty acids and glycerol.
Triglycerides are excellent storage molecules for several reasons:
High energy yield per gram due to many C–H bonds
Insolubility in water, preventing osmotic effects in cells
Compact structure, allowing efficient energy storage in small volumes
Release of metabolic water when oxidised, important in desert animals
Phospholipids
Phospholipids are key structural components of cell surface membranes and internal membranes such as those of mitochondria and chloroplasts. Their structure differs from triglycerides because one fatty acid is replaced by a phosphate group, forming a molecule with both hydrophobic and hydrophilic regions.
Hydrophilic: Water-attracting, interacting with water molecules.
The phosphate head is hydrophilic and charged, while the two fatty acid tails are hydrophobic, meaning they repel water. This creates an amphipathic molecule essential for membrane formation.
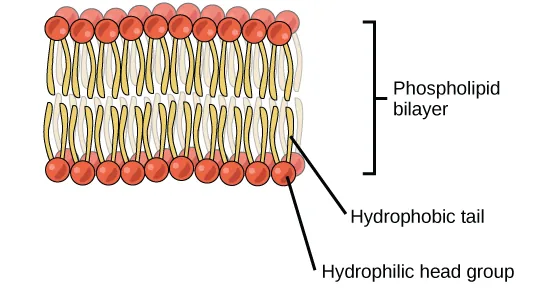
The phospholipid bilayer forms with hydrophilic phosphate heads facing aqueous environments and hydrophobic fatty acid tails sequestered inside. This architecture explains selective permeability and provides the matrix for membrane proteins. Source.
Phospholipids are arranged into a phospholipid bilayer, where:
Hydrophobic tails face inward, avoiding water
Hydrophilic heads face the aqueous cytoplasm and extracellular fluid
A flexible, partially permeable membrane is formed
This bilayer functions by:
Acting as a barrier to water-soluble substances
Allowing non-polar molecules to diffuse through
Providing a matrix for membrane proteins, receptors and channels
Phospholipids are also involved in:
Cell signalling, forming signalling molecules when hydrolysed
Membrane fluidity, influenced by the level of fatty acid saturation
Cholesterol
Cholesterol is a small, lipid-based steroid alcohol found in cell membranes of animals. Its structure consists of four hydrocarbon rings, making it compact and hydrophobic except for one small hydrophilic group.
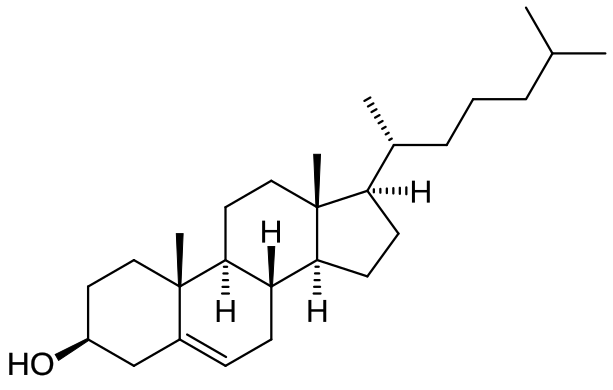
Cholesterol comprises four fused hydrocarbon rings with a single –OH group, explaining its insertion among phospholipid tails while aligning its hydroxyl near polar heads. This structure underpins cholesterol’s role in regulating membrane fluidity and reducing permeability. Source.
Cholesterol has several essential functions:
Regulates membrane fluidity by preventing phospholipid molecules from packing too closely at low temperatures or becoming too fluid at high temperatures
Stabilises the phospholipid bilayer due to interactions between its hydrophobic rings and phospholipid tails
Reduces membrane permeability to water and charged particles
Acts as a precursor for steroid hormones, bile salts and vitamin D
Lipid Synthesis and Hydrolysis
Triglycerides and phospholipids are synthesised and broken down in cells through enzyme-controlled condensation and hydrolysis reactions.
EQUATION
—-----------------------------------------------------------------
Triglyceride formation (esterification) = Glycerol + 3 Fatty acids → Triglyceride + 3 H₂O
Glycerol = Three-carbon alcohol forming ester bonds
Fatty acid = Hydrocarbon chain forming ester bonds
H₂O = Water, released during condensation
—-----------------------------------------------------------------
Enzymes such as lipases catalyse hydrolysis, particularly during digestion. In contrast, condensation occurs in smooth endoplasmic reticulum, where triglycerides and phospholipids are assembled and packaged for transport.
Key stages of lipid synthesis:
Fatty acids and glycerol are activated and aligned by enzymes
Ester bonds form through condensation
Lipids are transported in vesicles or combined with proteins to form lipoproteins for movement in blood
Structure–Function Relationships
The OCR specification emphasises the link between lipid structure and biological role. These core relationships include:
Triglycerides: long hydrocarbon chains store chemical energy and are insoluble, ideal for storage
Phospholipids: hydrophilic heads and hydrophobic tails allow bilayer and membrane formation
Cholesterol: rigid ring structure controls membrane fluidity and maintains stability
Lipids therefore contribute to energy storage, insulation, waterproofing, membrane structure and cell communication, making them fundamental in A-Level Biology understanding.
FAQ
Ester bonds are stable because they form strong covalent links between the hydroxyl group of glycerol and the carboxyl group of fatty acids, making them resistant to spontaneous breakdown at cellular pH.
However, digestive enzymes such as lipases catalyse hydrolysis, lowering the activation energy so water can split the ester bond. This allows controlled lipid digestion without compromising cell membranes.
Saturated fatty acids pack tightly due to their straight chains, increasing van der Waals forces and reducing membrane fluidity.
Unsaturated fatty acids contain double bonds that introduce kinks, spacing phospholipids further apart and increasing fluidity. The greater the number of double bonds, the more fluid the membrane becomes.
Phospholipids have two hydrophobic tails, making cylindrical molecules that pack neatly into bilayers.
This contrasts with single-tailed lipids, which are cone-shaped and favour micelle formation. Bilayers also provide:
A continuous hydrophobic barrier
A two-layer structure suitable for membrane proteins
Greater stability across large surface areas
Cholesterol fills gaps between phospholipid tails, restricting their movement and decreasing space for polar molecules to diffuse through.
Its rigid ring structure limits phospholipid flexibility, making the hydrophobic core denser and less penetrable to ions and small hydrophilic substances.
Triglycerides contain a higher proportion of hydrogen atoms relative to oxygen, creating many C–H bonds that release large quantities of ATP when oxidised.
Their long hydrocarbon chains undergo extensive oxidation in aerobic respiration, generating more reduced coenzymes than carbohydrates. This results in a greater ATP yield per gram.
Practice Questions
Question 1 (2 marks)
Describe the difference between saturated and unsaturated fatty acids and explain how this difference affects their physical properties.
Question 1 (2 marks)
Award 1 mark for each correct point:
Saturated fatty acids have no carbon–carbon double bonds, whereas unsaturated fatty acids have one or more double bonds. (1 mark)
Saturated fatty acids form straight chains and pack closely together, making them solid at room temperature, while unsaturated fatty acids have kinks and are more likely to be oils at room temperature. (1 mark)
Question 2 (5 marks)
Triglycerides, phospholipids and cholesterol each contribute to membrane structure and function. Compare their structures and explain how each lipid type helps maintain membrane properties.
Question 2 (5 marks)
Award up to 5 marks from the following points:
Triglycerides have three fatty acids bonded to glycerol; phospholipids have two fatty acids and a phosphate group; cholesterol has a four-ring structure with a small hydrophilic group. (1 mark)
Phospholipids form a bilayer with hydrophobic tails inside and hydrophilic heads facing out, creating a partially permeable membrane. (1 mark)
Cholesterol fits between phospholipid tails, reducing permeability and preventing them from packing too closely. (1 mark)
Cholesterol regulates membrane fluidity at different temperatures. (1 mark)
Triglycerides are mainly storage molecules but may be found in membranes in small amounts and provide hydrophobic barriers. (1 mark)
Accept correctly stated equivalents.

