OCR Specification focus:
‘Neutrophils and antigen-presenting cells use cytokines, opsonins, phagosomes and lysosomes to destroy pathogens; examine and draw cells from blood smears.’
Phagocytes are specialised white blood cells that form a crucial component of the non-specific immune response, recognising, engulfing and destroying pathogens to protect the body from infection.
Structure and Types of Phagocytes
Phagocytes are a diverse group of leucocytes (white blood cells) responsible for engulfing and digesting pathogens through the process of phagocytosis. There are two key types relevant to the OCR specification: neutrophils and antigen-presenting cells (APCs) such as macrophages and dendritic cells.
Neutrophils
Neutrophils are the most abundant type of phagocyte and are the body’s first line of cellular defence against invading microorganisms.
They are small, highly mobile cells that circulate in the blood and migrate rapidly to sites of infection.
Their nucleus is multi-lobed, aiding flexibility and allowing them to squeeze through capillary walls in a process called diapedesis.
The cytoplasm contains numerous lysosomes, filled with hydrolytic enzymes that digest ingested pathogens.
Lysosome: A membrane-bound organelle containing digestive enzymes (lysozymes) that break down proteins, lipids, and other biological molecules.
Neutrophils have a short lifespan, typically surviving only a few days, and die after phagocytosing several pathogens, forming part of pus in infected tissues.
Antigen-Presenting Cells (APCs)
Macrophages and dendritic cells act as long-lived phagocytes with a dual role in both non-specific and specific immune responses.
Macrophages originate as monocytes in the blood, which migrate into tissues and differentiate.
After engulfing a pathogen, macrophages process and present antigens on their surface bound to major histocompatibility complex (MHC) class II molecules.
Dendritic cells function similarly but are particularly efficient at activating T lymphocytes in the adaptive immune response.
Antigen-Presenting Cell (APC): A cell that processes antigens from pathogens and displays them on its surface using MHC molecules to activate T lymphocytes.
This antigen presentation links the innate and adaptive branches of immunity.
Mode of Action of Phagocytes
The mechanism of phagocytosis follows several coordinated stages:
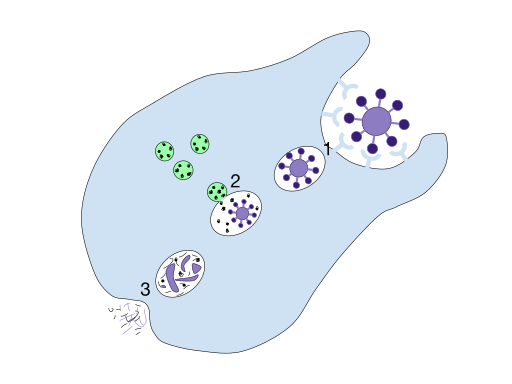
A labelled diagram of phagocytosis, showing pathogen recognition and engulfment, phagosome formation, fusion with lysosomes to form a phagolysosome, and exocytosis of residual bodies. Use it to consolidate the sequence before students learn antigen presentation. The image’s scope matches OCR expectations for non-specific destruction of pathogens. Source.
1. Pathogen Recognition
Phagocytes identify pathogens using cell surface receptors that recognise non-self antigens or are enhanced by molecules called opsonins.
Opsonin: A molecule (such as an antibody or complement protein) that binds to the surface of a pathogen, marking it for easier recognition and ingestion by phagocytes.
Opsonins coat the pathogen’s surface.
Common examples include immunoglobulin G (IgG) and complement proteins C3b.
This process is known as opsonisation.
2. Pathogen Engulfment
Once recognised, the phagocyte’s plasma membrane extends around the pathogen, enclosing it within a phagosome.
Phagosome: A membrane-bound vesicle formed when a phagocyte engulfs a pathogen.
The phagosome ensures that the pathogen remains isolated from the cytoplasm, preventing damage to the host cell.
3. Fusion with Lysosomes
The phagosome moves deeper into the cytoplasm and fuses with one or more lysosomes, forming a phagolysosome.
Lysozymes within the lysosome digest the pathogen by hydrolysing its macromolecules.
Reactive oxygen species (ROS) and nitric oxide may also be produced to enhance destruction.
The pathogen is broken down into harmless residues, including amino acids, sugars and lipids, which can be recycled or expelled.
4. Digestion and Exocytosis
After digestion, the remaining indigestible material (known as residual bodies) is removed from the cell by exocytosis.
In neutrophils, this marks the end of their life cycle, as they undergo apoptosis.
In macrophages, useful components may be retained, and the antigenic fragments are transported to the cell surface.
5. Antigen Presentation and Immune Activation
Antigenic peptides are combined with MHC class II proteins and displayed on the APC’s membrane surface.
This antigen-MHC complex is recognised by T helper cells (CD4⁺ lymphocytes), initiating the specific immune response.
Interleukins (a type of cytokine) are secreted by activated T cells, stimulating other immune cells such as B lymphocytes and further phagocytes.
This communication between immune cells is known as cell signalling, which ensures a coordinated and effective immune defence.
Cytokine: A small protein released by immune cells that acts as a signalling molecule to regulate inflammation and immune responses.
Key Features of Phagocytic Function
Rapid Response
Phagocytes act within minutes of infection, providing immediate defence before the adaptive immune response develops. Their mobility allows them to move quickly to damaged or infected tissues.
Chemotaxis
Phagocytes are chemotactic, meaning they are attracted by chemical signals such as histamine, cytokines, and pathogen-associated molecular patterns (PAMPs). This guides them precisely to infection sites.
Coordination with Other Defences
Inflammation increases blood flow and capillary permeability, facilitating phagocyte entry.
Complement proteins enhance phagocytosis through opsonisation and direct pathogen lysis.
Interferons released from virus-infected cells can activate macrophages to destroy infected cells.
Observation and Examination
Students are required to examine and draw phagocytes from blood smears, recognising neutrophils by their multi-lobed nuclei and granular cytoplasm. Macrophages appear larger with a single, kidney-shaped nucleus and clear cytoplasm.
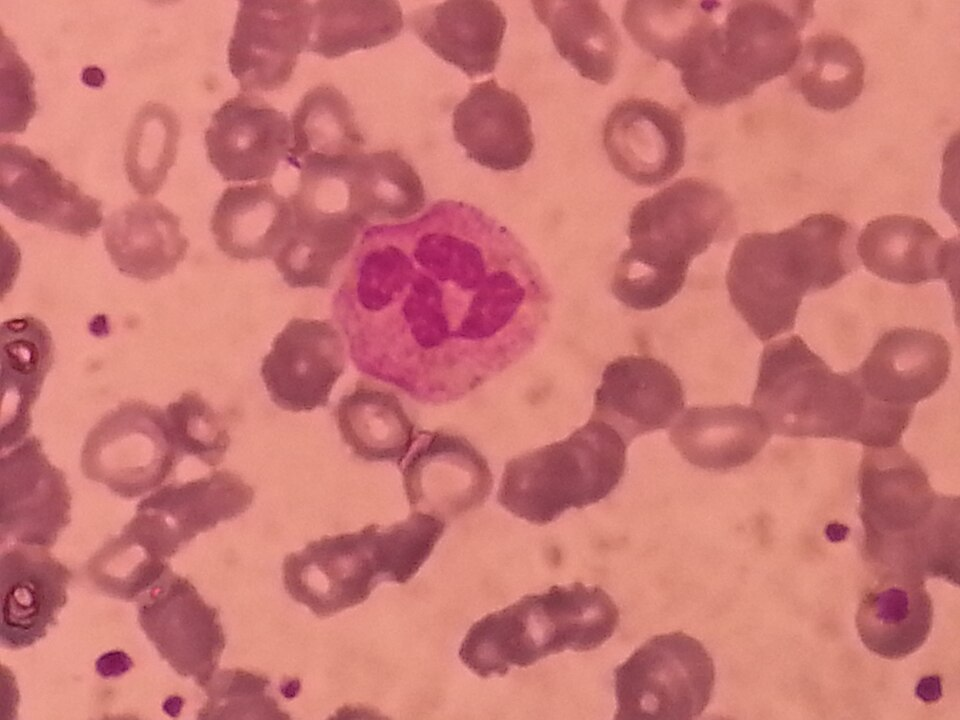
A Wright-stained peripheral blood smear with a neutrophil (centre) showing the characteristic multi-lobed nucleus and fine cytoplasmic granules. Erythrocytes and platelets appear in the background, which is normal for smears (additional content not required by the syllabus but helpful for orientation). Use this visual when teaching smear recognition and sketching. Source.
When stained appropriately (for example, with Wright’s or Giemsa stain), these cellular structures become visible, allowing comparison of their relative size and morphology.
Summary of Key Components
Phagocytes are white blood cells responsible for engulfing and destroying pathogens.
Neutrophils act rapidly but have a short lifespan.
Macrophages and dendritic cells act as antigen-presenting cells, bridging innate and adaptive immunity.
Key processes include opsonisation, phagosome formation, lysosomal digestion, and antigen presentation.
Cytokines and interleukins enable communication between immune cells, ensuring an orchestrated immune response.
FAQ
Phagocytes identify non-self antigens using specialised pattern recognition receptors (PRRs) that bind to pathogen-associated molecular patterns (PAMPs) such as bacterial cell wall components or viral RNA.
Self cells display distinctive self markers called major histocompatibility complex (MHC) class I molecules, which prevent healthy cells from being targeted. When cells lose these markers (e.g. virus-infected or cancerous cells), phagocytes and other immune cells may identify them as abnormal and destroy them.
If digestion is incomplete, some pathogens can survive inside the phagocyte, leading to chronic infection or disease spread.
For example:
Mycobacterium tuberculosis can prevent phagosome–lysosome fusion, persisting within macrophages.
The immune system may form granulomas, isolating infected macrophages to prevent further spread.
Incomplete destruction may also lead to persistent inflammation, which can damage surrounding tissues over time.
Neutrophils are designed for rapid response rather than long-term defence. They contain a high concentration of digestive enzymes and reactive oxygen species, which are toxic even to the cell itself.
Once neutrophils engulf several pathogens, they undergo apoptosis to prevent tissue damage.
Macrophages, in contrast, can survive for weeks to months, continuously engulfing debris and pathogens and acting as antigen-presenting cells to sustain immune coordination.
Cytokines act as chemical messengers, coordinating immune activity and inflammation.
Key effects include:
Recruiting more phagocytes to the infection site (chemotaxis).
Activating T helper cells to stimulate the adaptive immune response.
Increasing vascular permeability, allowing immune components to access infected tissues.
Promoting fever to slow pathogen reproduction.
Different cytokines (e.g. interleukin-1, interleukin-6, tumour necrosis factor) have specialised but overlapping roles in immune regulation.
Common stains include Wright’s stain, Giemsa stain, and Leishman’s stain. These stains highlight nuclear and cytoplasmic features, making identification easier under the microscope.
Neutrophils: show a multi-lobed purple nucleus and pale pink cytoplasm with fine granules.
Eosinophils (though not phagocytes): stain with distinctive orange-red granules, aiding differentiation.
Monocytes/macrophages: appear larger with a kidney-shaped nucleus and clear cytoplasm.
These staining techniques allow students to accurately distinguish and draw phagocytic cells for microscopy-based examination tasks.
Practice Questions
Question 1 (2 marks)
Describe the role of lysosomes in the destruction of pathogens by phagocytes.
Mark scheme:
Lysosomes fuse with the phagosome to form a phagolysosome (1 mark)
They contain hydrolytic enzymes (lysozymes) that break down the pathogen’s macromolecules (1 mark)
Question 2 (5 marks)
Explain how antigen-presenting cells (APCs) activate the specific immune response after phagocytosis of a pathogen.
Mark scheme:
After digestion, antigenic peptides from the pathogen are displayed on the APC surface (1 mark)
Antigens are bound to MHC class II molecules and presented on the cell membrane (1 mark)
T helper (CD4⁺) cells recognise the antigen–MHC complex using their specific receptors (1 mark)
Binding stimulates the release of interleukins (cytokines) from activated T helper cells (1 mark)
These cytokines activate B lymphocytes and other immune cells, coordinating the specific immune response (1 mark)

