OCR Specification focus:
‘Antibodies are proteins with specific binding sites; outline actions of opsonins, agglutinins and antitoxins; compare active versus passive, natural versus artificial immunity; introduce autoimmune disease (e.g. arthritis).’
Antibodies and immune mechanisms are key to the body’s specific immune response, enabling precise pathogen recognition, neutralisation and long-term protection through immunological memory and specialised cell coordination.
Structure and Function of Antibodies
Antibodies, also called immunoglobulins (Ig), are globular proteins produced by B lymphocytes that bind specifically to antigens on pathogens or toxins. They circulate in the blood and tissue fluids, forming an essential part of the adaptive immune system.
Antigen: Any molecule or part of a pathogen that is recognised as foreign by the immune system, triggering an immune response.
Each antibody has a quaternary protein structure, composed of four polypeptide chains — two identical heavy chains and two identical light chains held together by disulphide bridges. The overall shape resembles a ‘Y’.
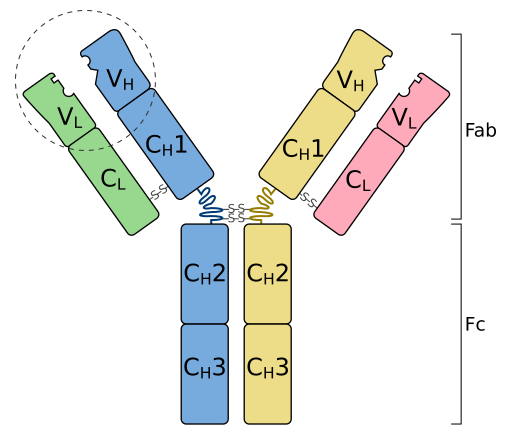
A labelled IgG basic unit showing two heavy (H) and two light (L) chains joined by disulphide bonds. The variable regions at the tips form the antigen-binding sites, while the constant regions make up the Fc and parts of Fab. The hinge region provides flexibility for binding antigens at different spacings. Source.
The variable region forms the antigen-binding site at the tips of the Y. Its unique amino acid sequence allows complementary binding to a specific antigen.
The constant region determines the antibody class and can bind to receptors on immune cells such as phagocytes.
The hinge region gives flexibility, allowing the antibody to bind to antigens spaced at varying distances.
Epitope: The specific part of an antigen to which an antibody binds.
The specificity of antigen–antibody binding follows a lock-and-key model, ensuring precise recognition of pathogens and effective targeting for destruction.
Antibody Production and Function
When a B lymphocyte is activated by contact with its specific antigen (often with T helper cell assistance), it undergoes clonal selection and clonal expansion to produce plasma cells and memory B cells. Plasma cells secrete antibodies into the bloodstream, while memory cells remain for long-term immunity.
Main Roles of Antibodies
Antibodies contribute to pathogen elimination through several mechanisms:
Neutralisation – antibodies bind to toxins or viral surface proteins, blocking their ability to attach to host cells.
Agglutination – antibodies cause pathogens to clump together, making them easier for phagocytes to engulf.
Opsonisation – antibodies act as opsonins, marking pathogens for phagocytosis.
Complement activation – antibody binding can trigger the complement cascade, leading to pathogen lysis.
Antitoxin activity – antibodies neutralise bacterial toxins by forming harmless complexes.
Each of these roles involves a different form of antibody action, reflected by specific antibody types such as opsonins, agglutinins, and antitoxins.
Types of Antibody Actions
Opsonins
Opsonins are antibodies or other molecules that bind to antigens on pathogen surfaces, enhancing phagocytosis by making pathogens easier to recognise by phagocyte receptors.
Opsonin: A molecule that binds to a pathogen and facilitates its uptake by phagocytes.
Common examples include IgG and IgM, which coat bacteria, allowing neutrophils and macrophages to efficiently engulf them. This process is vital for removing extracellular bacteria.
Agglutinins
Agglutinins are antibodies that bind to multiple pathogens simultaneously, forming large antigen–antibody complexes. These clumps (agglutinates) are too large to enter host cells and are easily removed by phagocytes.
This prevents pathogens from spreading and enhances their clearance.
Agglutination is particularly effective against bacteria and viruses in body fluids.
Agglutination: The clumping together of pathogens caused by antibodies binding multiple antigens at once.
Antitoxins
Antitoxins are antibodies that neutralise toxins released by pathogens. They bind directly to the toxin molecules, preventing them from interacting with host tissues.
For example, in diphtheria or tetanus, antitoxins prevent tissue damage caused by bacterial toxins.
The toxin–antitoxin complex is then removed by phagocytosis or filtration through the liver and kidneys.
Classes of Antibodies
There are five main classes (isotypes) of antibodies, each with a specialised role:
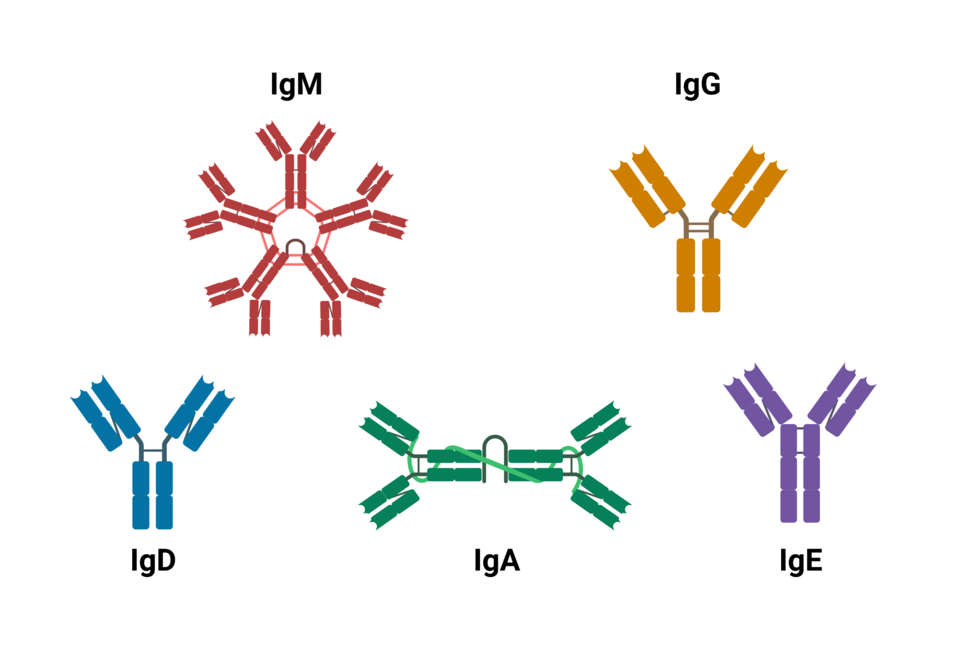
A clean, labelled comparison of major antibody isotypes (IgM, IgG, IgA, IgE, IgD). Shapes reflect common forms such as pentameric IgM and dimeric secretory IgA. Some diagrams may include subclasses (e.g., IgG1–IgG4), which are not required by OCR, but the core isotype overview matches the syllabus. Source.
IgM – the first antibody produced; effective at agglutination and complement activation.
IgG – the most abundant antibody in blood; provides long-term immunity and crosses the placenta.
IgA – found in secretions such as saliva, tears and mucus; protects epithelial surfaces.
IgE – involved in allergic responses; triggers histamine release from mast cells.
IgD – found on B cell membranes; involved in activation of B lymphocytes.
These structural and functional differences enable antibodies to defend against pathogens in various environments and physiological contexts.
Immunity: Active and Passive, Natural and Artificial
The immune system can acquire immunity through several distinct pathways. Understanding these classifications is key to interpreting immune protection and vaccine effects.
Active Immunity
Active immunity develops when the body produces its own antibodies after exposure to an antigen.
Natural active immunity arises from infection and recovery.
Artificial active immunity occurs after vaccination, where exposure to a safe form of the antigen induces immunity.
Active Immunity: Immunity produced by the body’s own immune response to antigen exposure.
Passive Immunity
Passive immunity results from receiving pre-formed antibodies rather than producing them.
Natural passive immunity occurs when maternal antibodies cross the placenta or are present in breast milk.
Artificial passive immunity is achieved through injection of antiserum containing antibodies, for example after snake bites.
Passive Immunity: Short-term protection provided by antibodies produced in another organism.
Active immunity is long-lasting but slower to develop, whereas passive immunity provides immediate but temporary protection. Both play vital roles in medical and natural defence contexts.
Autoimmune Diseases
Sometimes the immune system fails to distinguish self from non-self, leading to an autoimmune response where antibodies attack the body’s own tissues.
Autoimmune Disease: A condition in which the immune system produces antibodies against self-antigens, damaging body tissues.
An example is rheumatoid arthritis, where autoantibodies target the synovial membranes of joints, causing inflammation, pain, and loss of mobility. Other autoimmune diseases include lupus and Type 1 diabetes.
Autoimmune conditions illustrate the importance of immune regulation and self-tolerance mechanisms that normally prevent harmful antibody production against host tissues.
FAQ
The antibody class is determined by the constant region of its heavy chain, which defines its structural form and biological role.
Each class has distinct functions:
IgM is the first produced and excellent at agglutination.
IgG provides long-term immunity and crosses the placenta.
IgA protects mucosal surfaces.
IgE mediates allergic responses.
IgD aids B cell activation.
Class switching occurs when activated B cells change the heavy chain gene expressed, allowing the same antigen specificity but a new effector function.
Monoclonal antibodies are identical antibodies produced in laboratories from a single B cell clone. They all recognise the same epitope on an antigen.
In contrast, natural immune responses produce polyclonal antibodies — a mixture from many B cell clones targeting different epitopes on one pathogen.
Monoclonal antibodies are valuable in:
Medical diagnostics (e.g., pregnancy tests, ELISA).
Targeted therapies (e.g., anti-cancer or antiviral treatments).
Their high specificity allows precise binding but also requires careful design to avoid immune reactions.
Allergies occur when IgE antibodies bind to otherwise harmless antigens, such as pollen or food proteins.
The process involves:
IgE binding to mast cells and basophils via their Fc receptors.
Upon re-exposure to the allergen, cross-linking of IgE on these cells causes histamine release.
Histamine increases blood vessel permeability and stimulates inflammation, producing symptoms like itching, swelling, and sneezing.
This overreaction of the immune system is termed a hypersensitivity response.
Certain antibodies, mainly IgG and IgM, trigger the classical pathway of the complement system when bound to antigens.
Steps include:
Binding of the antibody’s Fc region to the C1 complex.
Activation of subsequent complement proteins, forming a membrane attack complex (MAC).
The MAC perforates pathogen membranes, leading to cell lysis.
Complement activation also enhances opsonisation and inflammation, amplifying immune efficiency.
The immune system maintains self-tolerance through several regulatory processes:
During lymphocyte development, self-reactive B and T cells are eliminated by clonal deletion in the bone marrow or thymus.
Some potentially self-reactive cells are inactivated rather than destroyed (a process called anergy).
Regulatory T cells (Tregs) suppress inappropriate immune responses against self-antigens.
Failure in these mechanisms can result in autoimmune diseases, such as rheumatoid arthritis or lupus.
Practice Questions
Question 1 (2 marks)
Describe how the structure of an antibody allows it to bind specifically to an antigen.
Mark Scheme:
1 mark for stating that the variable region of the antibody has a specific shape or amino acid sequence.
1 mark for explaining that this region is complementary to the shape of the antigen’s epitope, allowing specific binding through hydrogen bonds or other interactions.
Question 2 (5 marks)
Explain how antibodies contribute to the destruction and removal of pathogens, including reference to at least two different mechanisms of antibody action.
Mark Scheme:
1 mark for describing neutralisation – antibodies bind to toxins or viral surface proteins, preventing pathogens from attaching to host cells.
1 mark for describing agglutination – antibodies bind to multiple pathogens, causing them to clump together for easier phagocytosis.
1 mark for describing opsonisation – antibodies act as opsonins, marking pathogens for recognition and uptake by phagocytes.
1 mark for stating that antibodies can activate the complement system, leading to pathogen lysis.
1 mark for overall coherence and accurate use of scientific terminology (e.g. antigen, phagocyte, Fc region).
Total: 5 marks

