OCR Specification focus:
‘Refine experimental design by suggesting specific improvements to procedures and apparatus to improve data quality.’
Introduction
Improving experimental design is fundamental to producing reliable, valid, and accurate results in A-Level Chemistry investigations. By refining methods, controlling variables, and optimising apparatus, chemists ensure that data collected reflects true experimental outcomes.
Understanding Experimental Design
A strong experimental design allows scientists to test hypotheses effectively and obtain data that can be meaningfully analysed. It involves careful planning, selection of appropriate apparatus, and control of variables to ensure precision and reproducibility.
Key Principles of Experimental Design
Validity: Ensuring that the experiment measures what it intends to measure.
Reliability: Achieved when results are consistent upon repetition.
Accuracy: The degree to which measurements are close to true values.
Precision: The consistency of repeated measurements under identical conditions.
Each of these principles must be considered when improving an existing design to raise overall data quality.
Identifying Weaknesses in Experimental Design
Before proposing improvements, it is vital to identify areas where an experiment’s design may limit data quality. Weaknesses can arise from:
Poorly controlled variables, leading to invalid conclusions.
Inaccurate measurements due to inappropriate apparatus.
Procedural flaws, such as inconsistent timing or sample handling.
Environmental factors, including temperature or pressure variations.
Insufficient repeats, reducing the reliability of the results.
Evaluating Apparatus Selection
Selecting apparatus with appropriate sensitivity and range is essential. For example:
Choosing a burette over a measuring cylinder for titration increases precision.
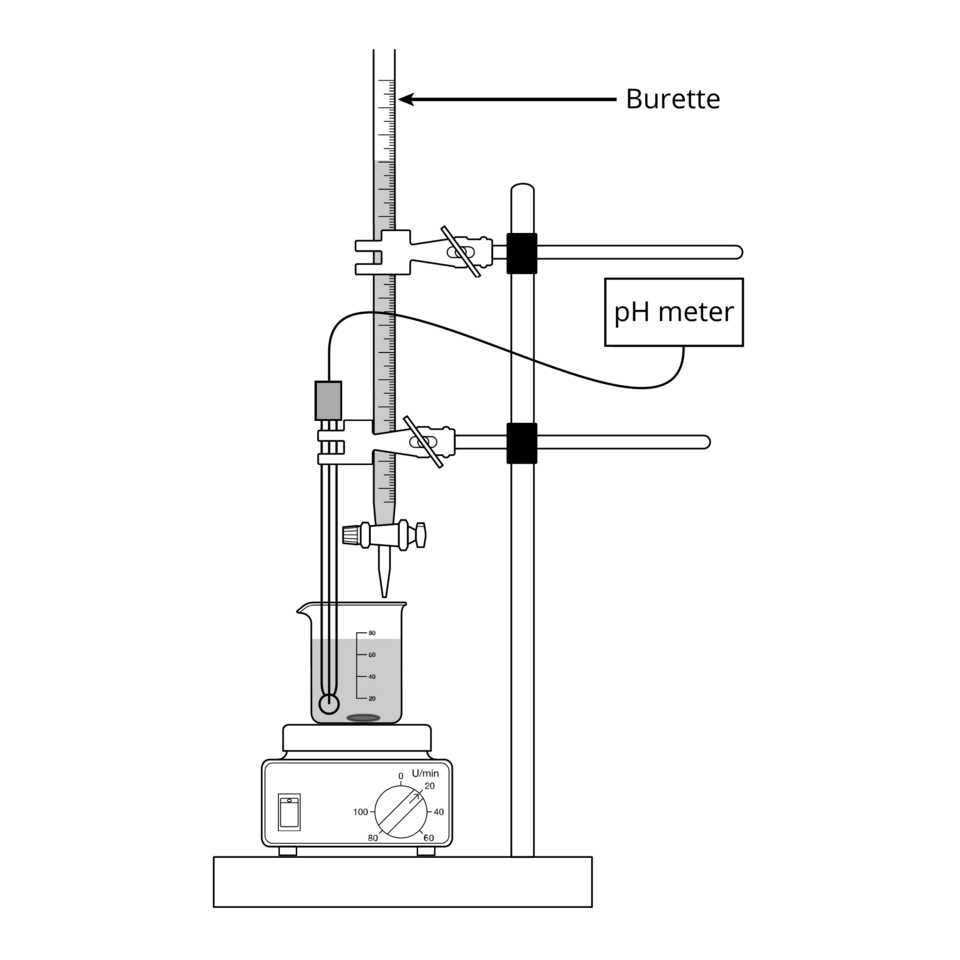
A clean, labelled diagram of an acid–base titration showing a burette delivering titrant into a conical flask. The orientation and labelling support accurate volume readings and safe swirling. This directly underpins apparatus selection for precision in volumetric analysis. Source
Using an analytical balance instead of a top-pan balance improves mass accuracy.
Such refinements directly influence measurement error and therefore data quality.
Measurement Error and Data Quality
Measurement error is an inevitable part of experimental work but can be minimised through careful design.
Measurement Error: The difference between a measured value and the true value of a quantity.
Errors may be systematic (affecting accuracy) or random (affecting precision). Identifying their sources allows for specific improvements in design, such as:
Calibrating apparatus before use to reduce systematic error.
Repeating measurements and calculating a mean to reduce random error.
Using equipment with smaller uncertainty values to enhance accuracy.
Strategies to Improve Experimental Design
1. Refining Procedures
Procedural improvements reduce variability and human error. Consider:
Standardising timings using digital timers.
Using consistent sample sizes or volumes throughout the experiment.
Maintaining constant environmental conditions, such as temperature using a thermostatically controlled water bath.

A laboratory circulating water bath used to maintain solutions at a set temperature. Stable thermal conditions improve reliability and repeatability by removing a major uncontrolled variable. Panel controls visible indicate setpoint and circulation—features that enhance uniform heating. Source
Ensuring complete mixing of reagents before measurement.
These refinements increase the repeatability of results, meaning the same person can reproduce them under identical conditions.
2. Optimising Apparatus Choice
Selecting the most appropriate apparatus is crucial for obtaining high-quality data:
Use digital thermometers or temperature probes for higher accuracy.
Employ volumetric pipettes for exact liquid measurement rather than syringes or measuring cylinders.
Choose data loggers for continuous and precise readings over manual observation.
Percentage Error (%) = (Uncertainty / Measured Value) × 100
Uncertainty = Half the smallest scale division of the measuring device
Measured Value = The recorded value from the experiment
Reducing percentage error by using better apparatus leads to improved accuracy and confidence in results.
3. Increasing Repeats and Sample Size
Performing multiple trials enables identification of anomalies and calculation of mean values, reducing the influence of random errors.
At least three repeats are recommended for each measurement.
Outliers should be identified and removed before calculating averages.
Larger sample sizes enhance the statistical reliability of conclusions.
4. Controlling Variables More Effectively
Uncontrolled variables can distort experimental outcomes. For valid results:
Keep all independent variables constant except the one being tested.
Use control experiments where the independent variable is omitted.
Implement automation (e.g., automated pipettes or stirring systems) to reduce human error in control maintenance.
5. Improving Data Collection and Recording
Refinements in data collection can greatly influence data reliability:
Record data with appropriate significant figures, matching the precision of the measuring device.
Use clear tables with headings, units, and uncertainties for transparency.
Employ software-based recording tools to minimise transcription errors.
Evaluating and Implementing Improvements
Improving experimental design is not only about identifying flaws but also about evaluating the impact of proposed changes on data quality. Each suggested improvement should be justified by explaining how it enhances accuracy, precision, or validity.
Example of Evaluation Process
Identify the limitation – e.g., “Temperature fluctuations affect reaction rate measurements.”
Propose improvement – e.g., “Use a thermostatically controlled water bath.”
Justify improvement – e.g., “Maintains constant temperature, reducing variation and improving reliability.”
This stepwise evaluation ensures that design refinements are evidence-based and purposeful.
Reliability, Repeatability, and Reproducibility in Design Improvements
Reliability: The degree to which results can be trusted because they are consistent and free from bias.
Repeatability: The ability to obtain the same results when the same person repeats the experiment under the same conditions.
Reproducibility: The ability to obtain the same results when a different person or group repeats the experiment under similar conditions.
When refining design:
Increase repeatability by improving consistency in technique and apparatus use.
Promote reproducibility by providing detailed methods and using standard equipment.
Assess reliability by comparing results with secondary data or established literature values.
Communicating Design Improvements
Clear and precise communication of proposed improvements is essential. When presenting an improved design:
Include revised apparatus lists and step-by-step procedural changes.
Explain how each change reduces uncertainty or error.
Indicate expected outcomes in terms of improved data precision or reliability.
Summary of Core Approaches for Refinement
Use apparatus with higher precision and lower uncertainty.
Control environmental factors to improve validity.
Increase repeats for better reliability.
Calibrate instruments to remove systematic errors.
Refine procedures to reduce human error.
Every change should aim to improve the overall data quality, ensuring that experimental conclusions are scientifically robust and credible.
FAQ
Improving accuracy focuses on reducing systematic errors so that results are closer to the true value. This can be achieved by calibrating instruments or using more suitable apparatus.
Improving precision, on the other hand, involves minimising random errors so that repeated measurements are closely grouped. Using equipment with smaller uncertainty values and maintaining consistent technique can help achieve this.
Environmental factors such as temperature, humidity, and light intensity can affect chemical reactions and measurements. To control them:
Use thermostatically controlled water baths or incubators.
Shield light-sensitive experiments with opaque containers.
Conduct experiments in enclosed rooms to reduce draughts or temperature variation.
By standardising these conditions, data reliability and reproducibility improve significantly.
Calibration ensures that measuring instruments give readings close to the true value by comparing them to known standards. For example, balances can be calibrated using certified weights, and pH meters with standard buffer solutions.
Regular calibration reduces systematic errors and ensures that subsequent results remain valid and traceable.
Automation reduces human error and enhances consistency in repetitive tasks such as titration, data logging, or temperature monitoring.
Examples include:
Automated burettes for precise titrant delivery.
Electronic timers or sensors for consistent reaction monitoring.
Data loggers for continuous and unbiased measurement collection.
These tools improve repeatability and reduce operator bias.
Pilot testing allows students to identify procedural flaws before conducting the full experiment. It highlights issues such as unclear steps, unsuitable apparatus, or timing difficulties.
Benefits include:
Refining measurement methods for better accuracy.
Identifying sources of error early.
Ensuring that all variables can be controlled effectively.
This preliminary step strengthens the final experimental design and enhances the validity of collected data.
Practice Questions
A student is investigating the effect of temperature on the rate of a chemical reaction. They record inconsistent results each time they repeat the experiment. Suggest two improvements to the experimental design that would help improve the reliability of their results. (2 marks)
(1) Use a thermostatically controlled water bath to maintain a constant temperature throughout the experiment.
(1) Repeat the experiment several times under identical conditions and calculate a mean value.
A student designs an experiment to determine the concentration of hydrochloric acid by titration with sodium hydroxide solution. They use a measuring cylinder to add the alkali, perform only one titration, and record their results without including uncertainties. Evaluate the limitations of this design and suggest improvements that would enhance the accuracy and reliability of the results. (5 marks)
(1) Identifies that a measuring cylinder is not sufficiently precise for volume measurement.
(1) Suggests using a burette or volumetric pipette for improved accuracy.
(1) Notes that performing only one titration reduces reliability; should repeat titrations until concordant results are obtained.
(1) Mentions omission of uncertainties or significant figures affects the reliability of reported data.
(1) Explains how suggested improvements (more precise apparatus, repeats, correct reporting) increase accuracy and reliability of data.

