OCR Specification focus:
‘Assess reliability through repeats and comparison with secondary data, considering repeatability and reproducibility in evaluation.’
Understanding Reliability, Repeatability and Reproducibility
Reliability, repeatability, and reproducibility are essential aspects of experimental evaluation in chemistry. They determine whether data can be trusted and whether conclusions drawn are scientifically valid. Reliable results strengthen the confidence in experimental findings, ensuring consistency across trials, individuals, and equipment.
Reliability in Experimental Work
Reliability refers to the overall trustworthiness of experimental results. Reliable experiments yield data that are consistent and credible when measurements are repeated or compared with those obtained from other sources. In OCR A-Level Chemistry, evaluating reliability involves assessing how well data align with theoretical predictions and independent results.
Factors influencing reliability include:
Consistency of measurements: Small variation between repeated readings increases reliability.
Appropriate control of variables: Controlled experimental conditions minimise extraneous influences.
Instrument calibration: Properly calibrated equipment ensures that readings are accurate and comparable.
Operator competence: Skilled use of apparatus and adherence to standard procedures reduce human error.
Reliable experiments are characterised by reproducible patterns, minimal anomalies, and agreement with established chemical principles.
Repeatability: Consistency Under Identical Conditions
Repeatability focuses on how consistent results are when the same person repeats an experiment using the same equipment and conditions.
Repeatability: The degree to which the same experimental results can be obtained by the same person using the same equipment and method under identical conditions.
Repeatability is often assessed through repeated trials. If the measured values are very similar, the data set is described as highly repeatable.
Key practices to ensure repeatability:
Maintain constant environmental conditions such as temperature or pressure.
Use the same sample size and reagents for all trials.
Record results to appropriate significant figures to maintain precision.
Repeat measurements multiple times and calculate mean values to minimise random error.
When repeatability is low, variability between results indicates potential random or systematic errors that must be identified and controlled.
Reproducibility: Agreement Across Different Conditions
Reproducibility assesses whether the same results can be achieved when the experiment is conducted by different people, using different equipment, or even in different laboratories.
Reproducibility: The degree to which the same experimental results can be obtained under changed conditions, such as by different operators, laboratories, or equipment.
Reproducibility evaluates the robustness of a method. If different teams can obtain comparable results, the procedure is likely reliable and free from major bias.
Ways to enhance reproducibility:
Clearly document methodology and apparatus details.
Use standardised procedures that others can follow precisely.
Compare results with secondary data from literature or other research groups.
Use inter-laboratory testing to confirm results under varied circumstances.
Reproducibility provides external validation for findings and helps ensure that results are not specific to a single experimenter or setup.
Relationship Between Repeatability, Reproducibility, and Reliability
Reliability depends on both repeatability and reproducibility. An experiment that produces similar results only when repeated by the same person may be repeatable but not necessarily reliable. True reliability requires that results are both repeatable and reproducible, indicating that they are consistent across multiple conditions and observers.
For instance, if a titration experiment consistently yields the same concentration when repeated by several students using different burettes, the method is both repeatable and reproducible, thus reliable.
Evaluating Data Reliability
In OCR A-Level Chemistry, students are expected to assess reliability by:
Performing repeats to detect anomalies and calculate mean results.
Comparing data with secondary sources such as literature values or published data.
Considering the precision of measurements and their agreement with accepted values.
Discussing how procedural factors (such as equipment sensitivity or operator error) influence reliability.
Reliable data sets exhibit:
Low standard deviation between repeated measurements.
Consistency with theoretical expectations.
Absence of significant anomalies that cannot be explained scientifically.
Compare measurements from different methods or sources to evaluate agreement, bias, and limits of agreement before citing conclusions.
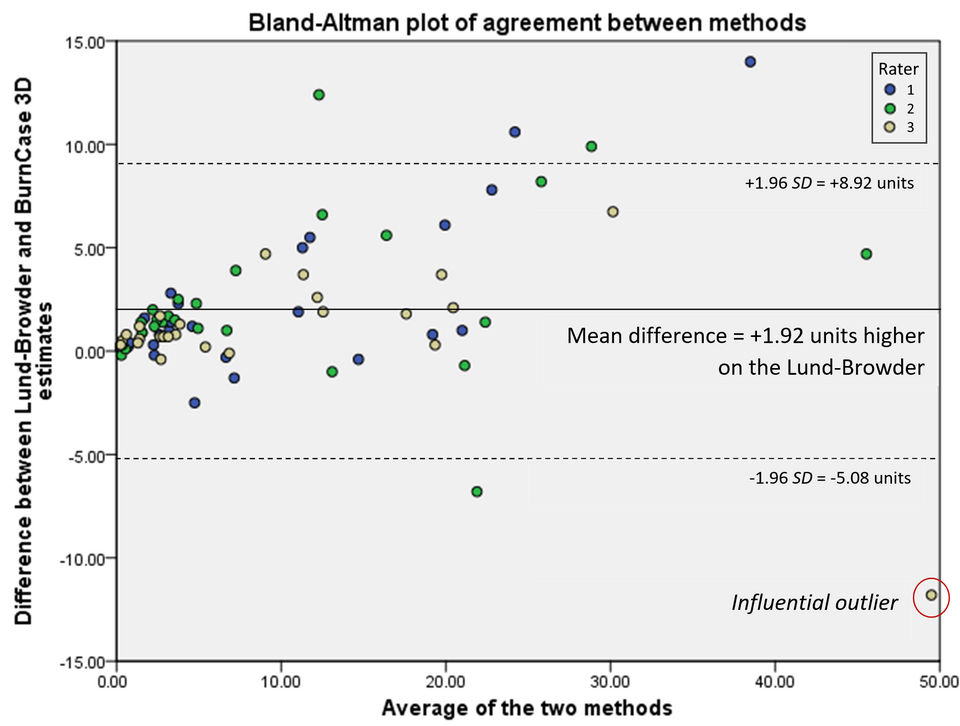
Bland–Altman plot showing mean difference (bias) and 95% limits of agreement for two measurement methods. Points outside the dashed limits indicate poor agreement and potential issues undermining reproducibility. This visual is appropriate when validating results against an alternative technique or literature values. Source
Importance of Secondary Data
Secondary data provides a reference point for assessing reproducibility and reliability. It includes results obtained from textbooks, research papers, or other laboratories. When primary data (student results) agree with secondary data within the expected range of experimental error, it suggests that both the procedure and data are reliable.
Chemists use secondary data to:
Validate methods across institutions.
Identify systematic errors if discrepancies arise.
Confirm that experimental design aligns with accepted practice.
Identifying Limitations in Repeatability and Reproducibility
When results vary beyond acceptable limits, it is essential to analyse the causes.
Possible factors include:
Instrumental error — poor calibration or malfunction.
Human error — inconsistent technique, parallax error, or incorrect timing.
Environmental fluctuations — temperature or humidity changes affecting reaction rates.
Uncontrolled variables — impurities or inconsistent reagent concentrations.
Addressing these issues improves both repeatability and reproducibility, thereby enhancing the experiment’s overall reliability.
Improving Reliability Through Experimental Design
To ensure data reliability, chemists incorporate strategies that minimise uncertainty and standardise procedures:
Perform at least three repeats for each measurement to identify anomalies.
Use precise measuring instruments (e.g. analytical balances, digital thermometers).
Calibrate instruments before and after experiments.
Keep detailed methodological notes for others to replicate.
Cross-check with literature values or peer data to verify findings.
Such practices contribute to a strong evidence base and demonstrate that results are both dependable and scientifically valid.
In interlaboratory or two-run studies, visualise repeatability and reproducibility using appropriate plots to separate within-lab and between-lab variation.
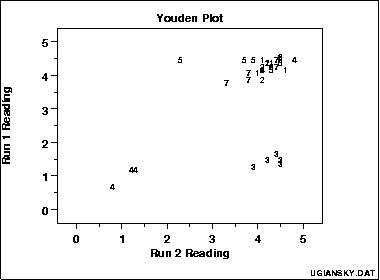
Youden plot with each symbol representing a laboratory’s two results (run 1 vs run 2). Departure from the 45° line indicates repeatability problems; systematic displacement across labs indicates reproducibility problems. This plot is recommended by NIST for analysing interlab data. Source
Quantitative Indicators of Reliability
Quantitative evaluation helps assess consistency in results numerically. Calculating mean, range, and standard deviation allows chemists to measure variation within repeated data. A low standard deviation indicates high repeatability, supporting the reliability of conclusions.
Additionally, expressing percentage uncertainty in measurements provides a clearer sense of data precision and highlights areas where improvements in method or apparatus could enhance reliability.
Integrating Reliability, Repeatability and Reproducibility in Evaluation
When evaluating practical results in examinations or investigations, always consider:
Whether results are repeatable under identical conditions.
Whether results are reproducible under different conditions.
How these aspects combine to determine overall reliability.
Whether secondary data supports your findings.
Any potential limitations or sources of error affecting these factors.
Incorporating these elements ensures that scientific evaluation meets the rigorous standards of OCR A-Level Chemistry and reflects authentic professional laboratory practice.
FAQ
Random errors cause unpredictable variations in measurements, often due to uncontrollable factors such as temperature changes or timing inaccuracies. They affect precision and can be reduced by repeating measurements and averaging results.
Systematic errors cause consistent deviations in one direction, usually due to calibration faults or incorrect technique. They affect accuracy and cannot be eliminated by repetition but can be identified by comparing results with known or secondary data.
Reliability can be measured using statistical methods such as calculating standard deviation or coefficient of variation.
A low standard deviation suggests high repeatability.
Comparing results to accepted values provides evidence of reliability.
Using error bars on graphs visually shows variability between repeats, helping to assess data quality.
Reproducibility ensures that findings are valid and not dependent on a specific set of conditions or experimenters.
If another laboratory using different apparatus and reagents can achieve similar results, it confirms that the conclusions are robust. This underpins the credibility of scientific studies and supports peer verification.
Improving reproducibility involves standardising methods and communication:
Use detailed, clearly written protocols with exact reagent specifications.
Ensure equipment calibration standards are consistent across laboratories.
Conduct pilot studies to test consistency before the full experiment.
Regularly compare data trends to detect anomalies early.
Control experiments are used to isolate the effect of the independent variable. By keeping all other factors constant, chemists can ensure that observed changes result only from the intended variable.
They:
Provide a baseline for comparison.
Reveal potential interference or contamination.
Strengthen the validity and reliability of the overall conclusions.
Practice Questions
An experiment is repeated three times by the same student using the same equipment and conditions. The results are very similar. What term is used to describe this consistency, and what does it indicate about the quality of the data? (2 marks)
1 mark: Identifies the term repeatability.
1 mark: Explains that it indicates high consistency and precision in the data under identical conditions.
A group of chemistry students from different schools perform the same titration experiment using different burettes and pipettes. Each group obtains slightly different results for the concentration of the acid.
(a) Explain how reproducibility and reliability can be assessed in this investigation.
(b) Suggest two factors that could cause differences in the results between schools. (5 marks)
(a)
1 mark: States that reproducibility is assessed by comparing results obtained by different people, equipment, or laboratories.
1 mark: Explains that results showing similar values indicate the experiment is reproducible.
1 mark: States that reliability is assessed by evaluating both repeatability and reproducibility together, showing consistent agreement with theoretical or secondary data.
(b)
1 mark: Mentions differences in equipment calibration or precision between schools.
1 mark: Mentions variation in technique or human error (e.g., reading burette levels differently).

