OCR Specification focus:
‘Apply IUPAC nomenclature with locants for substituted arenes, e.g. 2,4-dinitromethylbenzene.’
Naming substituted aromatic compounds requires a precise understanding of IUPAC rules, correct use of locants, and recognition of priority groups to ensure accurate, unambiguous nomenclature in aromatic chemistry.
Principles of Aromatic Nomenclature
Naming substituted aromatic compounds centres on applying systematic IUPAC rules to benzene-derived structures. Benzene acts as the parent molecule whenever it is the principal structural feature. Substituents are named as prefixes, and locants indicate their positions around the ring to avoid ambiguity. The specification emphasises the ability to apply IUPAC nomenclature with locants, including names such as 2,4-dinitromethylbenzene, illustrating correct ordering, numbering, and substituent designation.
When Benzene Is the Parent Name
Benzene remains the parent structure when the aromatic ring is the highest-priority functional component. This requires careful assessment of substituents and their functional class.
Parent compound: The principal structural unit selected as the core of the molecule to which substituents are attached in IUPAC naming.
A single sentence must follow definition blocks before continuing with any structured list or further definitions. When benzene is the parent, substituents are listed alphabetically and prefixed with the appropriate locants.
Numbering the Aromatic Ring
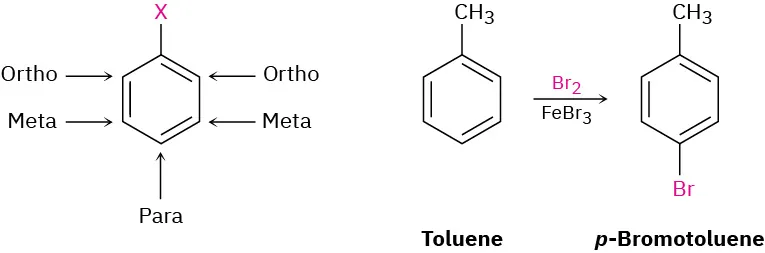
These examples illustrate how aromatic rings are numbered to achieve the lowest possible set of locants when multiple substituents are present. One structure uses toluene as a retained parent name, extending beyond strict benzene nomenclature while reinforcing numbering principles. Source
Numbering begins at the carbon attached to the substituent with the highest priority. The direction chosen (clockwise or anticlockwise) must give the lowest possible set of locants.
Key rules for assigning locants include:
Ensure the lowest set of numbers when multiple substituents are present.
Assign position 1 to the substituent with the highest functional priority (e.g. carboxylic acids outrank alkyl groups).
If two substituents are identical, begin numbering at one of them and proceed to give the next substituent the lowest possible number.
Avoid unnecessary numbering: monosubstituted benzene does not require locants.
Substituent Names and Alphabetical Order
Substituents attached to the benzene ring are written as prefixes. Their names follow standard IUPAC practice, and alphabetical ordering is based on the first letter of the substituent name, ignoring any multiplicative prefixes such as di-, tri-, or tetra-.
Common substituent prefixes include:
Methyl-
Nitro-
Bromo-
Amino-
Hydroxy-
Alphabetical ordering examples:
Amino (A) comes before bromo (B).
Bromo (B) comes before methyl (M).
Nitro (N) comes before methyl (M).
A normal sentence such as this ensures separation before further structured information is introduced. Students must remember that alphabetical ordering refers to substituent names, not locants.
Use of Locants in Names
This diagram compares disubstituted benzene arrangements and shows the equivalence between ortho/meta/para naming and the 1,2 / 1,3 / 1,4 locant system. The labels emphasise how relative position determines the prefix, independent of substituent identity. Source
Locants indicate the exact positions of substituents around the aromatic ring. They must appear immediately before the relevant substituent name and be separated by commas when multiple numbers apply.
Important rules for using locants:
Use commas between numbers: 2,4-dinitromethylbenzene.
Use hyphens between numbers and substituent names: 4-nitrobenzoic acid.
When identical substituents appear multiple times, use multiplicative prefixes: di-, tri-, tetra-.
Always ensure locants correspond to the lowest possible numbering pattern.
Priority of Functional Groups
When the benzene ring contains a substituent that is recognised as a functional group with naming priority, benzene may no longer be used as the parent name. Instead, the compound is named as a substituted derivative of the functional group.
Examples of substituents with higher priority than simple groups:
Carboxylic acid (–COOH) → benzoic acid becomes the parent structure.
Aldehyde (–CHO) → benzaldehyde becomes the parent structure.
Nitro groups (–NO2) and halogens do not override benzene as the parent and remain prefixes.
Functional group priority directly affects the numbering scheme, as the carbon bearing the principal functional group is always carbon-1.
Special Cases in Aromatic Nomenclature
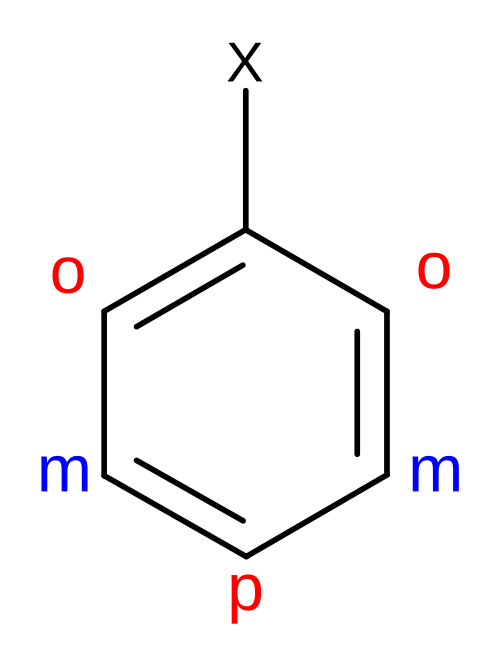
This schematic shows the ortho, meta, and para positions relative to a single substituent on a benzene ring. It is a positional reference diagram designed to support rapid identification of disubstituted aromatic arrangements. Source
Certain aromatic compounds have traditional names that are still accepted under IUPAC rules, but students must be able to apply systematic names as required by the specification. These special cases arise from historically common structures.
Common traditional parent names include:
Phenol (C6H5OH) — the OH group defines carbon-1.
Aniline (C6H5NH2) — the amino group defines carbon-1.
Toluene (C6H5CH3) — the methyl group defines carbon-1.
A normal sentence is useful here to emphasise that, although traditional names are allowed, systematic numbering and substitution rules still apply to all substituted derivatives.
Nitro and Alkyl Substitution Patterns
Substituted nitrobenzenes and alkylbenzenes frequently appear in OCR A-Level contexts. Their naming follows all previous rules but emphasises clarity in locants and alphabetical order.
When naming compounds such as 2,4-dinitromethylbenzene, students must apply the following steps:
Identify benzene as the parent ring.
Recognise methyl and nitro substituents.
Apply alphabetical order: dinitro precedes methyl when multiplicative prefixes are ignored.
Assign locants based on the lowest possible numbering.
Combine substituent names with locants to produce an unambiguous final name.
Multiple Substituents and Complex Prefixes
Compounds with three or more substituents require careful organisation. In these cases:
Select the numbering direction that yields the lowest set of locants.
Alphabetise substituents by base name, ignoring multiplicative prefixes.
Apply numbering consistently even if substituents differ widely in chemical nature.
Use commas and hyphens precisely to maintain strict IUPAC format.
Aromatic nomenclature becomes more complex as substituent patterns increase, but the core principles remain consistent across all substituted arenes.
FAQ
Numerical locants are preferred because they remove ambiguity and work for all substitution patterns, including trisubstituted and more complex aromatic rings.
They allow:
Clear identification of exact carbon positions
Consistency across all aromatic compounds
Compatibility with alphabetical ordering rules
Ortho, meta and para are limited to disubstituted benzene rings and are mainly used in simpler or traditional naming contexts.
When no substituent has higher functional priority, carbon 1 is chosen to give the lowest possible set of locants overall.
If two numbering options give identical locant sets:
The substituent that comes first alphabetically is given the lower number
This rule ensures consistent naming when functional group priority alone does not resolve numbering.
No, multiplicative prefixes are ignored when alphabetising substituent names.
For example:
Dinitro is alphabetised under “n”, not “d”
Trichloro is alphabetised under “c”, not “t”
Only the base substituent name is considered when ordering prefixes in the final IUPAC name.
Traditional names are retained because they are widely used, unambiguous, and recognised by IUPAC.
They are acceptable when:
The functional group defines carbon 1 clearly
Substitution patterns can still be numbered correctly
Students must still apply standard locant and alphabetical rules when naming substituted derivatives of these compounds.
No, under IUPAC rules there should be only one correct systematic name.
Different names may appear if:
Incorrect numbering is chosen
Alphabetical order is ignored
Traditional naming is mixed incorrectly with systematic naming
Following numbering, priority, and alphabetical rules consistently ensures a single unambiguous name.
Practice Questions
Give the full IUPAC name of the aromatic compound shown, which contains a benzene ring with two nitro groups at positions 2 and 4 and a methyl group at position 1.
(2 marks)
Correct identification of benzene as the parent compound: 1 mark
Correct name with appropriate locants and substituent order: 2,4-dinitromethylbenzene: 1 mark
A benzene ring has three substituents: a methyl group, a nitro group, and a bromo group.
The methyl group is taken as carbon 1, the nitro group is on carbon 3, and the bromo group is on carbon 4.
a) Explain how the numbering of the benzene ring is determined in this compound. (2 marks)
b) State the full IUPAC name of the compound, including correct locants and substituent order. (3 marks)
(5 marks)
a) Numbering of the benzene ring (2 marks)
Carbon 1 is assigned to the methyl group as the chosen reference substituent: 1 mark
Ring is numbered to give the lowest possible set of locants to the remaining substituents (3 and 4 rather than higher alternatives): 1 mark
b) IUPAC name (3 marks)
Correct use of locants for all substituents (1-methyl, 3-nitro, 4-bromo or equivalent): 1 mark
Correct alphabetical order of substituents in the name (bromo before methyl before nitro): 1 mark
Fully correct IUPAC name: 4-bromo-1-methyl-3-nitrobenzene: 1 mark

