OCR Specification focus:
‘Describe nitration, halogenation with carriers, and Friedel–Crafts alkylation/acylation to form C–C bonds to rings.’
Electrophilic substitution reactions allow aromatic rings to react while retaining aromaticity, forming substituted arenes through characteristic reactions involving powerful electrophiles under carefully controlled conditions.
Electrophilic Substitution in Arenes
Arenes, such as benzene, undergo a distinctive class of reactions known as electrophilic substitution. These reactions replace a hydrogen atom on the aromatic ring with another atom or group, while preserving the stability of the delocalised π system.
Electrophilic substitution: A reaction in which an electrophile replaces a hydrogen atom in an aromatic ring without destroying the aromatic π system.
A key feature of these reactions is that addition reactions are avoided because they would disrupt the delocalisation that stabilises the aromatic ring. Instead, substitution allows aromaticity to be retained.
General Features of Electrophilic Substitution
Electrophilic substitution reactions of arenes share several common characteristics:
The aromatic ring acts as an electron-rich nucleophile due to its delocalised π electrons
A strong electrophile is required to attack the ring
A catalyst or reagent is often needed to generate the electrophile
Reaction conditions are carefully controlled to prevent multiple substitution
Electrophile: An electron-pair acceptor attracted to regions of high electron density.
These reactions typically proceed more slowly than reactions of alkenes, reflecting the extra stability of the aromatic π system.
Nitration of Benzene
Nitration introduces a nitro group (–NO₂) into the benzene ring and is one of the most important electrophilic substitution reactions.
Reagents: concentrated nitric acid and concentrated sulfuric acid
Conditions: 50–60 °C to limit substitution to one nitro group
Product: nitrobenzene
Sulfuric acid acts as a catalyst, generating the powerful electrophile needed for reaction.
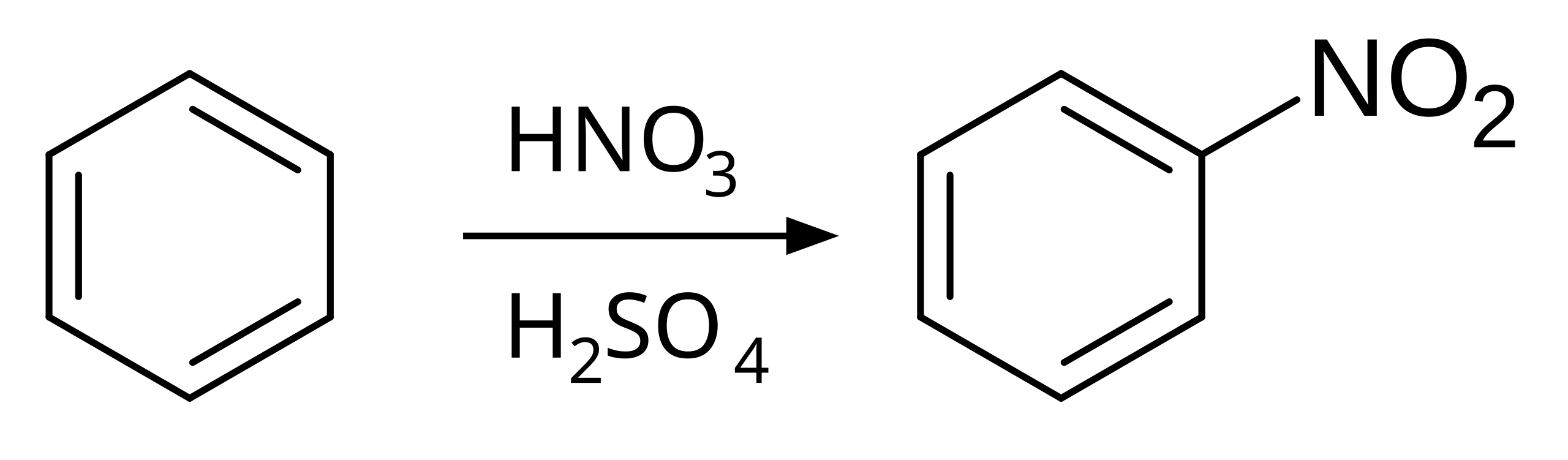
Nitration is an electrophilic substitution in which a nitro group replaces a hydrogen atom on benzene. Concentrated nitric acid and sulfuric acid are used to generate suitable nitrating conditions. This diagram shows the overall transformation rather than the stepwise mechanism. Source
Overall nitration of benzene
C₆H₆ + HNO₃ → C₆H₅NO₂ + H₂O
The temperature must be controlled carefully; higher temperatures increase the risk of dinitration and oxidation of the ring.
Halogenation of Benzene with a Halogen Carrier
Benzene does not react directly with halogens without a catalyst. Halogen carriers are required to generate the electrophile.
Common halogens: chlorine and bromine
Catalysts: AlCl₃, FeCl₃, AlBr₃, or FeBr₃
Products: chlorobenzene or bromobenzene
The halogen carrier reacts with the halogen molecule to form a positively charged halogen electrophile.
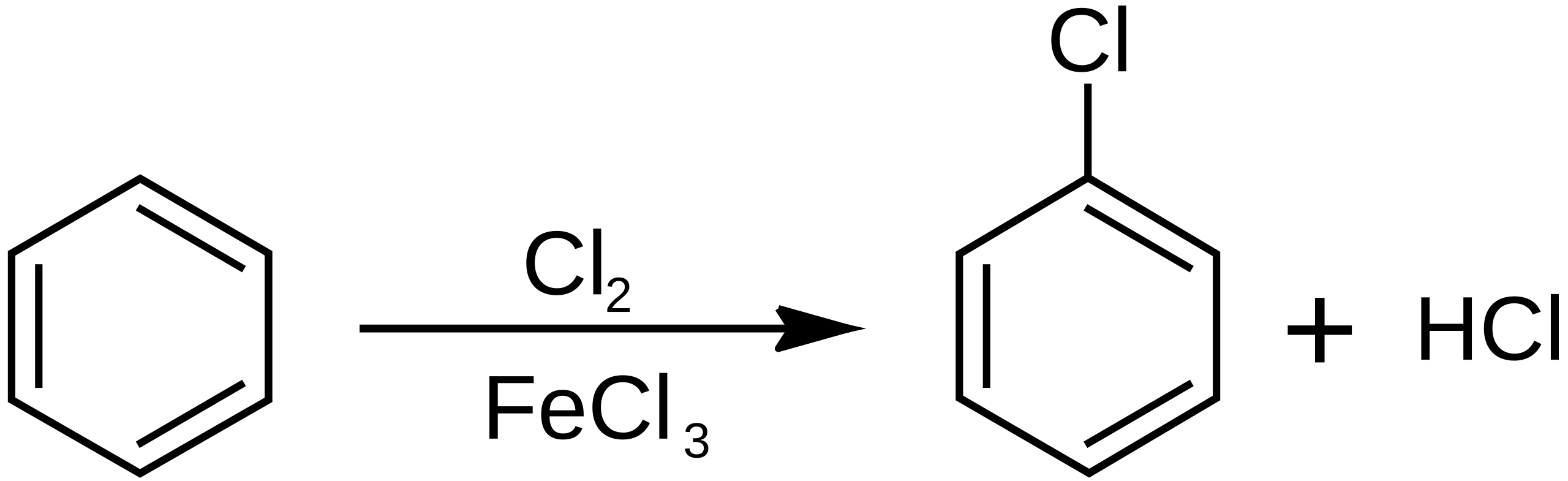
Halogenation of benzene is an electrophilic substitution in which a halogen replaces a ring hydrogen. A halogen carrier polarises the halogen molecule to generate a strong electrophile. The scheme illustrates the overall reaction rather than mechanistic detail. Source
Halogen carrier: A catalyst that polarises a halogen molecule to generate a reactive halogen electrophile.
Chlorination of benzene
C₆H₆ + Cl₂ → C₆H₅Cl + HCl
Unlike alkenes, benzene does not decolourise bromine water, highlighting its resistance to addition reactions and supporting the delocalised model.
Friedel–Crafts Alkylation
Friedel–Crafts alkylation introduces an alkyl group into the aromatic ring, forming a new carbon–carbon bond.
Reagents: haloalkane and AlCl₃ catalyst
Product: alkylbenzene
The aluminium chloride reacts with the haloalkane to generate a carbocation electrophile.
This reaction demonstrates how arenes can be used to build larger organic molecules, but it has important limitations:
Multiple substitution is common because alkyl groups activate the ring
Rearrangement of carbocations can occur, leading to unexpected products
Friedel–Crafts alkylation
C₆H₆ + RCl → C₆H₅R + HCl
Because of these issues, this reaction is not always suitable for controlled synthesis.
Friedel–Crafts Acylation
Friedel–Crafts acylation introduces an acyl group (–COR) onto the aromatic ring and is often preferred to alkylation.
Reagents: acyl chloride and AlCl₃ catalyst
Product: aryl ketone
The acyl chloride reacts with aluminium chloride to form an acylium ion, a stable electrophile.
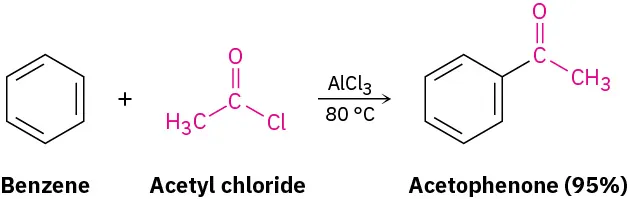
Friedel–Crafts acylation forms a new carbon–carbon bond between an aromatic ring and an acyl group. An acyl chloride reacts with AlCl₃ to generate the electrophile required for substitution. Temperature and yield information shown exceed OCR syllabus requirements. Source
Acylium ion: A positively charged electrophile, RCO⁺, formed from an acyl chloride in the presence of AlCl₃.
Friedel–Crafts acylation
C₆H₆ + RCOCl → C₆H₅COR + HCl
This reaction has significant advantages:
Only one substitution occurs because the acyl group deactivates the ring
No carbocation rearrangement occurs
The reaction is more controllable and predictable
Importance in Organic Synthesis
Electrophilic substitution reactions of arenes are essential tools in organic chemistry. They allow:
Controlled substitution while retaining aromaticity
Introduction of functional groups for further reactions
Formation of carbon–carbon bonds using Friedel–Crafts reactions
The resistance of benzene to addition reactions, combined with its ability to undergo substitution, is central to its chemistry and industrial usefulness.
FAQ
Benzene contains a delocalised π system that gives it extra stability compared with alkenes.
Addition reactions would break this delocalisation and produce a less stable, non-aromatic product. As a result, benzene resists addition and instead undergoes substitution, which preserves the aromatic π system after the reaction is complete.
AlCl₃ acts as a Lewis acid because it accepts an electron pair.
In Friedel–Crafts reactions, AlCl₃ accepts a lone pair from a halogen or acyl chloride, helping to generate a strong electrophile. This electron-pair acceptance is essential for activating the reagent so that substitution can occur on the benzene ring.
Nitration is exothermic and becomes more vigorous at higher temperatures.
If the temperature is too high, multiple nitration can occur, producing dinitro or trinitro compounds. Excessive heating can also increase oxidation of the benzene ring, reducing yield and producing unwanted by-products.
Alkenes have a localised C=C double bond with high electron density, allowing direct reaction with halogens.
Benzene’s electrons are delocalised and stabilised, making it less reactive. A halogen carrier is needed to polarise the halogen molecule and generate a sufficiently strong electrophile to attack the aromatic ring.
Friedel–Crafts alkylation involves carbocation intermediates.
These carbocations can rearrange to form more stable structures, leading to unexpected products. Alkyl groups also activate the benzene ring, increasing the likelihood of multiple substitution, whereas acyl groups deactivate the ring and prevent further substitution.
Practice Questions
Benzene reacts with concentrated nitric acid in the presence of concentrated sulfuric acid to form nitrobenzene.
Explain why sulfuric acid is needed in this reaction.
(2 marks)
Award one mark for each of the following points:
Sulfuric acid acts as a catalyst / generates the electrophile.
Sulfuric acid produces a more reactive electrophile (e.g. NO₂⁺) from nitric acid.
Maximum 2 marks.
Friedel–Crafts acylation is an important electrophilic substitution reaction of benzene.
(a) State the reagents and conditions used for Friedel–Crafts acylation.
(b) Explain why Friedel–Crafts acylation usually results in only one substitution on the benzene ring.
(c) State one advantage of Friedel–Crafts acylation compared with Friedel–Crafts alkylation.
(5 marks)
(a) Reagents and conditions (2 marks)
Award one mark for each correct point:
Use of an acyl chloride and a Lewis acid catalyst such as AlCl₃.
Anhydrous conditions / moderate temperature (or equivalent suitable condition).
(b) One substitution only (2 marks)
Award one mark for each correct point:
The acyl group deactivates the benzene ring.
The deactivated ring is less reactive towards further electrophilic substitution.
(c) Advantage over alkylation (1 mark)
Award one mark for one of the following:
No carbocation rearrangement occurs.
Multiple substitution is avoided.
Reaction is more controlled or predictable.
Maximum 5 marks.

