OCR Specification focus:
‘Explain water solubility of carboxylic acids using hydrogen bonding between molecules and water.’
Introduction
Carboxylic acids display distinctive physical properties due to their ability to form extensive hydrogen bonding, influencing their solubility in water, intermolecular forces, and observable behaviour.
Understanding Hydrogen Bonding in Carboxylic Acids
Carboxylic acids contain the carboxyl functional group (–COOH), which plays a crucial role in determining their physical properties. The key to their behaviour lies in the strong intermolecular forces arising from hydrogen bonding, a type of permanent dipole–dipole attraction formed when a hydrogen atom is bonded to highly electronegative atoms such as oxygen.
Hydrogen Bonding: A strong dipole–dipole attraction between a hydrogen atom bonded to an electronegative atom (such as O, N, or F) and a lone pair on another electronegative atom.
Hydrogen bonding in carboxylic acids occurs both between the acid molecules themselves and between acid molecules and water, directly linking to their solubility as required by the OCR specification focus.
Key Structural Features Enabling Hydrogen Bonding
Polar C=O bond: The carbonyl oxygen is highly electron-rich, creating strong dipoles.
O–H bond: The hydroxyl hydrogen is strongly δ⁺, making it highly attractive to nearby lone pairs.
Two electronegative oxygens: Allow each molecule to act as both hydrogen bond donor and acceptor, increasing overall interactions.
These features give rise to distinctive behaviours compared to most other organic compounds of similar molar mass.
Hydrogen Bonding Between Carboxylic Acid Molecules
Carboxylic acids commonly form dimers, in which two acid molecules are held together by two hydrogen bonds.
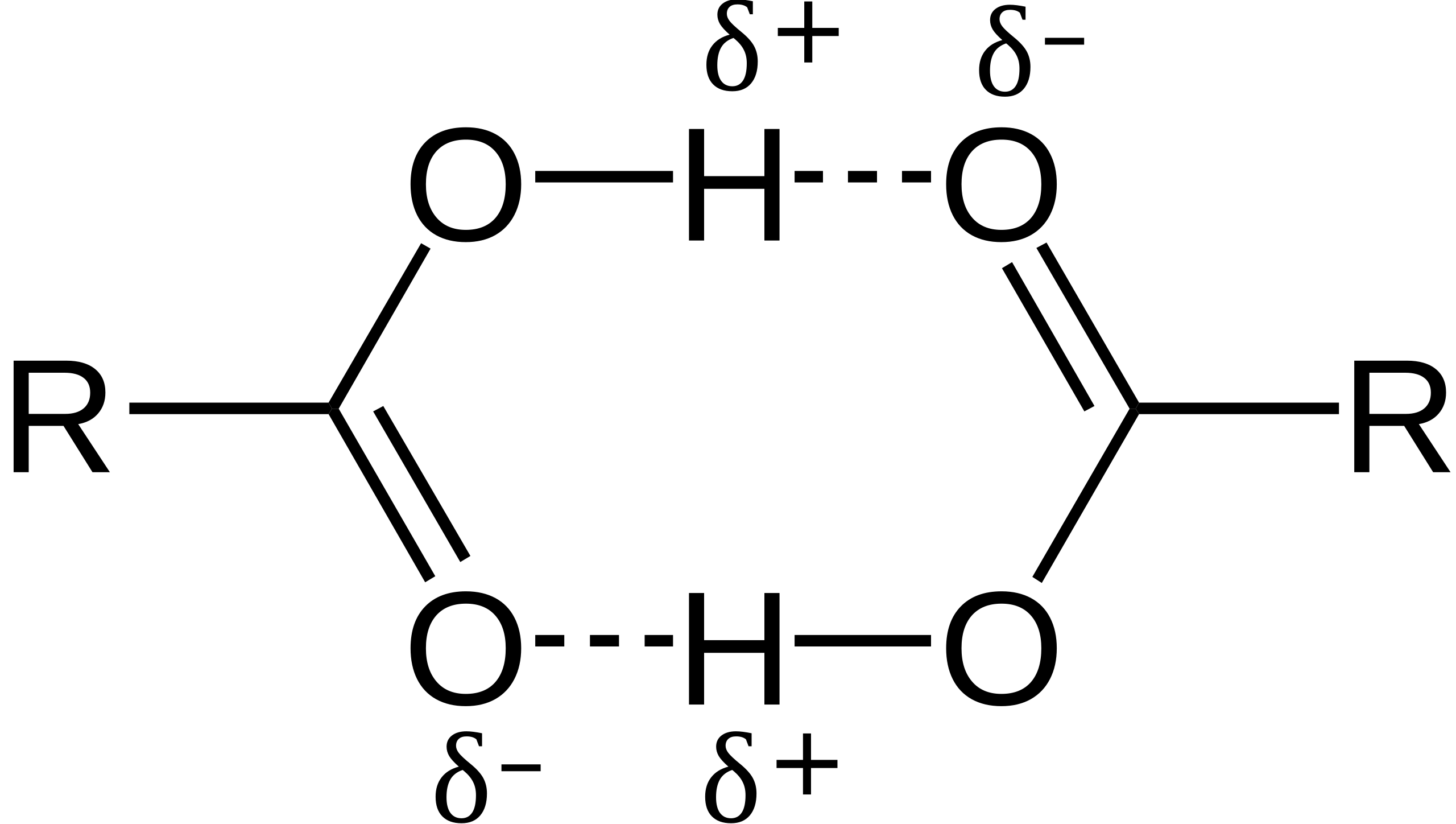
This diagram shows two carboxylic acid molecules forming a cyclic dimer via two hydrogen bonds between the O–H group and the carbonyl oxygen. The δ⁺ and δ⁻ labels highlight molecular polarity, which supports hydrogen bond formation. Source
This dimerisation significantly increases intermolecular attraction.
Features of Dimer Formation
Each molecule donates one hydrogen bond and accepts one.
Dimers increase the effective size of the molecules, raising boiling points.
Dimers exist particularly in non-polar solvents or the gaseous state.
Bullet points summarising effects of dimerisation:
Higher boiling points than expected from molecular mass.
Lower volatility, making many acids pungent but less prone to evaporation.
Stronger intermolecular forces overall due to paired hydrogen bonds.
Between definition and any equation, include at least one normal sentence. This ensures clarity and meets the formatting requirements.
Hydrogen Bonding with Water: Basis of Solubility
The OCR specification states that students must “explain water solubility of carboxylic acids using hydrogen bonding between molecules and water,” so this section focuses on that exact requirement.
Interaction of Carboxylic Acids with Water
Carboxylic acids are soluble in water because they form strong hydrogen bonds with water molecules.
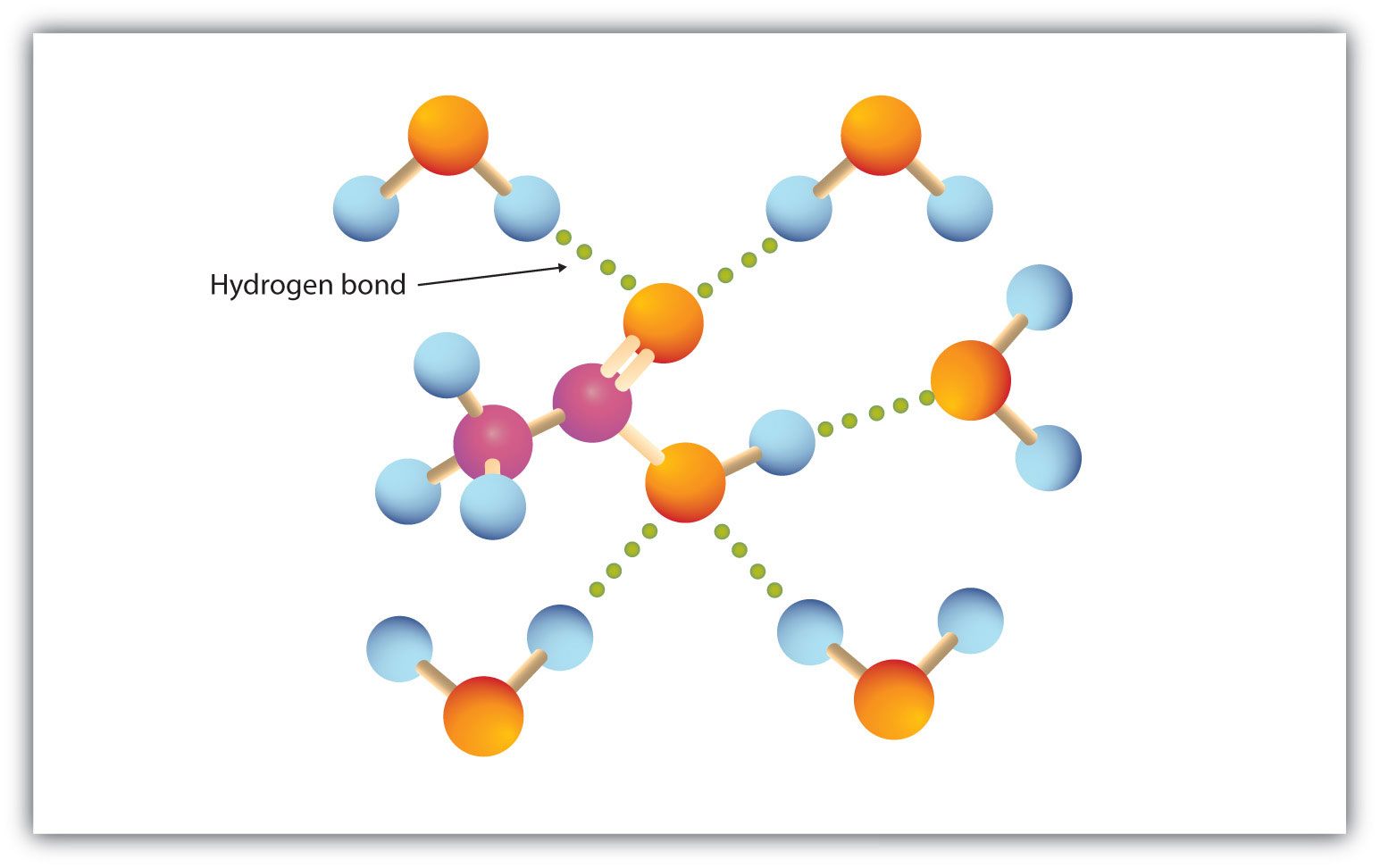
The diagram illustrates hydrogen bonds between a carboxylic acid molecule and surrounding water molecules, showing how both the carbonyl oxygen and hydroxyl hydrogen interact with water. Additional water–water hydrogen bonding is shown for context, although this exceeds the syllabus requirement. Source
Both oxygens in the –COOH group take part in these interactions.
The carbonyl oxygen accepts hydrogen bonds from water molecules.
The hydroxyl hydrogen donates hydrogen bonds to water.
These combined interactions cause small carboxylic acids to dissolve readily.
Hydrogen Bond Donor: A species that provides a hydrogen atom involved in hydrogen bonding (typically O–H, N–H, or F–H).
A normal sentence must appear before any additional definition or equation blocks to maintain clear separation.
Hydrogen Bond Acceptor: A species that provides a lone pair that forms the other half of a hydrogen bond.
Factors Affecting Solubility in Water
Smaller carboxylic acids (up to around four carbon atoms) are highly soluble because the hydrogen bonding interactions outweigh the hydrophobic effects of the carbon chain. As chain length increases, solubility decreases.
Important factors influencing solubility:
Ability to form multiple hydrogen bonds with water.
Polarity of the functional group, enhancing interactions with polar solvents.
Carbon chain length, which reduces solubility as non-polar character grows.
Bullet-Point Summary of Hydrogen Bonding Effects
Increased solubility for short-chain acids due to strong hydrogen bonds with water.
Decreased solubility for long-chain acids because hydrophobic chain dominates.
Enhanced interactions between acid and solvent compared with aldehydes or ketones.
Contribution of both oxygens in the carboxyl group to aqueous hydrogen bonding.
Comparing Carboxylic Acids with Other Organic Families
Carboxylic acids are generally more soluble in water than many other organic compounds of similar molar mass due to their dual hydrogen bonding capability. Both alcohols and carbonyl compounds form hydrogen bonds to some extent, but not as extensively as carboxylic acids.
Key Differences
Alcohols have one O–H group, whereas carboxylic acids have a carbonyl oxygen as an additional hydrogen bond acceptor.
Aldehydes and ketones cannot donate hydrogen bonds, making their solubility lower.
The ability to both donate and accept hydrogen bonds strengthens interactions with water.
Role of Hydrogen Bonding in Physical Properties
Hydrogen bonding affects not only solubility but also the physical behaviour of carboxylic acids.
Important points include:
Higher boiling points than comparable aldehydes and ketones.
Lower volatility due to strong intermolecular forces.
Distinctive liquid properties, such as viscosity and strong odours.
These features trace back to the combination of hydrogen bonding between acid molecules and hydrogen bonding to water.
FAQ
Carboxylic acids form stronger hydrogen bonds because the carboxyl group contains two oxygen atoms rather than one.
The carbonyl oxygen is highly electron-rich and is a particularly strong hydrogen bond acceptor, while the hydroxyl group can donate a hydrogen bond.
This dual donor–acceptor capability allows carboxylic acids to form multiple, reinforcing hydrogen bonds, including stable cyclic dimers, which alcohols cannot form.
As the carbon chain length increases, the non-polar hydrocarbon portion of the molecule becomes more dominant.
This hydrophobic region disrupts interactions with water, reducing the overall effect of hydrogen bonding from the carboxyl group.
Eventually, the energy gained from hydrogen bonding is insufficient to overcome the unfavourable interactions between the hydrocarbon chain and water.
In aqueous solution, carboxylic acids do not predominantly exist as dimers.
Water molecules compete effectively for hydrogen bonding with the carboxyl group, breaking up acid–acid hydrogen bonds.
As a result, hydrogen bonding with water is favoured over dimer formation in solution, increasing solubility.
Carboxylic acids can both donate and accept hydrogen bonds, whereas aldehydes and ketones can only accept hydrogen bonds.
This allows carboxylic acids to form stronger and more numerous interactions with water molecules, leading to greater solubility despite similar molecular masses.
Strong hydrogen bonding between carboxylic acid molecules reduces their volatility.
More energy is required to separate the molecules during evaporation because of these strong intermolecular attractions.
As a result, carboxylic acids tend to evaporate less readily and often have sharp, persistent odours rather than being highly volatile liquids.
Practice Questions
Explain why ethanoic acid is soluble in water.
(2 marks)
Award marks as follows:
1 mark for stating that ethanoic acid forms hydrogen bonds with water.
1 mark for explaining that the carboxyl (–COOH) group contains oxygen atoms that allow hydrogen bonding (either donation from O–H or acceptance at C=O).
Maximum 2 marks.
Carboxylic acids have higher boiling points and greater water solubility than aldehydes of similar molecular mass.
Explain these differences in terms of hydrogen bonding and molecular structure.
(5 marks)
Award marks as follows:
1 mark for recognising that carboxylic acids can form hydrogen bonds, while aldehydes cannot donate hydrogen bonds.
1 mark for explaining that carboxylic acids contain an O–H bond, enabling hydrogen bond donation.
1 mark for explaining that the carbonyl oxygen in carboxylic acids acts as a hydrogen bond acceptor.
1 mark for linking hydrogen bonding between carboxylic acid molecules (dimer formation) to higher boiling points.
1 mark for explaining that hydrogen bonding between carboxylic acids and water increases solubility.
Maximum 5 marks.
Credit explanations that clearly link structure to intermolecular forces and physical properties.

