OCR Specification focus:
‘Describe reactions with metals, oxides, alkalis, and carbonates under aqueous conditions.’
Carboxylic acids show characteristic reactions with a range of basic and reactive species in aqueous conditions, providing important evidence for their acidity and utility in synthesis.
Reactions of Carboxylic Acids with Metals and Bases
Carboxylic acids are weak Brønsted–Lowry acids that donate a proton to suitable bases, producing salts that contain the carboxylate ion. Their predictable reactivity with metals, metal oxides, alkalis, and carbonates forms a key foundation for understanding acid–base chemistry in organic systems. All reactions occur under aqueous conditions, as required by the OCR specification, because water facilitates proton transfer and stabilises ionic products.
Fundamental Acid–Base Behaviour of Carboxylic Acids
When carboxylic acids react with basic substances, the reactions typically involve proton transfer from the –COOH group to a species capable of accepting a proton.
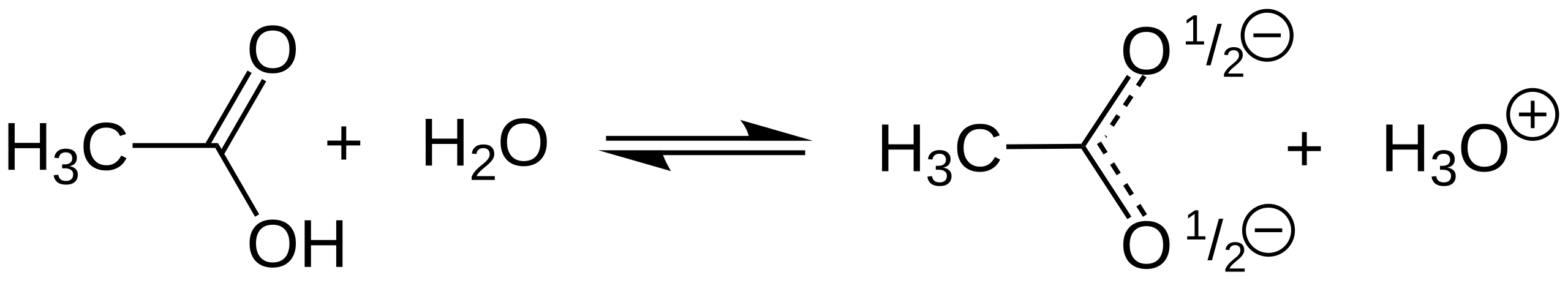
This diagram shows ethanoic acid donating a proton to water, forming ethanoate and hydronium ions. It illustrates the proton transfer that underpins neutralisation reactions of carboxylic acids with alkalis and other bases. Source
Brønsted–Lowry Acid: A species that donates a proton (H⁺) to another species.
The behaviour of carboxylic acids reflects this definition closely, forming the conjugate base carboxylate ion (RCOO⁻) upon reaction with various bases. This underpins the reactions described across this subsubtopic.
Carboxylic acids also display redox behaviour when reacting with reactive metals, in which hydrogen gas is produced as the metal is oxidised and the acid is reduced.
Reaction with Metals
Reactive metals such as magnesium or calcium undergo a redox reaction with carboxylic acids.

This image shows a metal reacting with an acid to release hydrogen gas, visible as effervescence, while forming a dissolved metal salt. The same observation applies when reactive metals react with carboxylic acids under aqueous conditions. Source
These reactions demonstrate the acids’ ability to release hydrogen ions that can be reduced to hydrogen gas.
Key features of reactions with metals:
Occur readily in aqueous conditions.
Produce a carboxylate salt and hydrogen gas.
Follow the general pattern characteristic of acids reacting with metals.
General reaction pattern:
Metal + Carboxylic acid → Metal carboxylate + Hydrogen gas
Metal–Acid Reaction (n/a) = Metal + 2 RCOOH → (RCOO)₂M + H₂
RCOOH = Carboxylic acid; the acidic species donating H⁺
(RCOO)₂M = Metal carboxylate salt formed
H₂ = Hydrogen gas produced
Hydrogen effervescence provides a visible sign of reaction progression, and the formation of metal carboxylate salts illustrates the ionisation of carboxylic acids.
Reaction with Metal Oxides
Metal oxides behave as basic oxides and neutralise carboxylic acids. The reaction is a straightforward acid–base neutralisation.
Key features:
Produces a carboxylate salt and water.
Does not involve redox processes.
Provides a clear demonstration of the neutralising ability of carboxylic acids.
Bullet-point overview:
Carboxylic acid donates H⁺ to oxide ion.
Oxide ion (O²⁻) accepts the proton, forming water.
Carboxylate ion pairs with metal cation to form the salt.
This neutralisation mirrors the behaviour of mineral acids, reinforcing that carboxylic acids, though weak, participate fully in classical acid–base reactions.
Reaction with Alkalis
Alkalis such as sodium hydroxide react quantitatively with carboxylic acids. Because alkalis contain hydroxide ions (OH⁻), proton transfer is complete and irreversible under aqueous conditions.
Key outcomes:
Produces a carboxylate salt and water.
Reaction goes to completion due to the strength of hydroxide as a base.
Essential for purification processes such as separating acids from organic mixtures.
Alkali: A soluble base that produces hydroxide ions (OH⁻) in aqueous solution.
Process outline:
Hydroxide ions accept the proton from the carboxylic acid.
Water forms as the neutral product.
Carboxylate ion pairs with the alkali cation (e.g., Na⁺, K⁺).
This reaction is widely used in organic synthesis to deprotonate carboxylic acids, enabling further conversions via their carboxylate salts.
Reaction with Carbonates and Hydrogencarbonates
Carboxylic acids react with carbonates (CO₃²⁻) and hydrogencarbonates (HCO₃⁻), producing carbon dioxide gas in addition to the expected salt and water.

The foam and bubbles show carbon dioxide gas being released when ethanoic acid reacts with a hydrogencarbonate. This is the characteristic observation for reactions between carboxylic acids and carbonates or hydrogencarbonates. Source
Key features:
Produces carboxylate salt, carbon dioxide, and water.
Effervescence of CO₂ provides qualitative evidence for the presence of an acid.
Reaction is slower than with alkalis but still proceeds readily.
Layered breakdown:
Carboxylic acid transfers a proton to carbonate or hydrogencarbonate.
Carbonic acid (H₂CO₃) forms transiently, decomposing into CO₂ and H₂O.
Carboxylate salt forms simultaneously.
Because carbon dioxide gas escapes from the reaction mixture, the process continues until all acid is consumed, a feature common to acid–carbonate reactions.
Comparing Reactivity Across Metals, Oxides, Alkalis, and Carbonates
Carboxylic acids show consistent acid–base reactivity across these groups, but each reaction type offers distinct observable features:
Metals: Redox process with hydrogen gas formation.
Metal oxides: Neutralisation leading to salt and water only.
Alkalis: Complete neutralisation with quantitative conversion to carboxylate salt.
Carbonates: Visible effervescence due to CO₂ gas release.
The stability of the carboxylate ion explains the reliability of these reactions. Resonance within the carboxylate group disperses negative charge, allowing salts to form readily and remain stable in aqueous environments.
Importance in Synthetic and Analytical Chemistry
The reactions of carboxylic acids with metals and bases underpin key practical techniques:
Formation of carboxylate salts provides intermediates for further transformations, including acyl chloride synthesis and ester formation.
Reactions with carbonates deliver rapid qualitative tests for carboxylic acid functional groups.
The broad similarity to mineral acid behaviour allows predictable acid–base behaviour without requiring specialised conditions.
These reactions, all performed under aqueous conditions, exemplify the characteristic reactivity of carboxylic acids as outlined in the OCR A-Level Chemistry specification.
FAQ
Carboxylic acids are weak acids, meaning they only partially ionise in aqueous solution.
This results in a lower concentration of hydrogen ions available to be reduced to hydrogen gas, so the reaction rate with metals is slower compared with strong acids such as hydrochloric acid.
The presence of the organic R group also slightly reduces the accessibility of the acidic proton compared with small inorganic acids.
Carboxylate salts contain charged ions, which interact strongly with polar water molecules.
Hydration of the carboxylate ion stabilises it in solution, preventing reformation of the acid.
Short-chain carboxylate salts are particularly soluble due to effective ion–dipole interactions, whereas salts with very long hydrocarbon chains may show reduced solubility.
Carbon dioxide is a gas and escapes from the reaction mixture as it forms.
This removal of a product shifts the equilibrium in favour of further reaction, ensuring the carboxylic acid continues to react until it is fully consumed.
This is why reactions with carbonates do not reach equilibrium under normal laboratory conditions.
The carboxylate ion is stabilised by delocalisation of negative charge over two oxygen atoms.
This resonance reduces charge density on any one atom, lowering the energy of the ion.
As a result, carboxylate salts form readily and are more stable than salts derived from many other weak acids.
Water allows acids and bases to dissociate into ions, enabling proton transfer.
It stabilises charged species such as carboxylate ions, hydroxide ions, and metal cations through hydration.
Without aqueous conditions, many of these reactions would be much slower or may not proceed to a detectable extent at all.
Practice Questions
Ethanoic acid reacts with aqueous sodium carbonate.
a) State two observations you would expect to see during this reaction.
(2 marks)
a)
Effervescence / bubbling observed (1 mark)
Gas identified as carbon dioxide OR formation of a salt and water mentioned (1 mark)
Maximum 2 marks
Carboxylic acids react with a range of bases under aqueous conditions.
Describe and explain the reactions of a carboxylic acid with:
a reactive metal
an alkali
a carbonate
Your answer should include the products formed and the type of chemistry involved.
(5 marks)
Reaction with a metal produces hydrogen gas and a metal carboxylate salt (1 mark)
Identifies this as a redox reaction OR mentions reduction of H⁺ to H₂ (1 mark)
Reaction with an alkali produces a carboxylate salt and water (1 mark)
Describes this as an acid–base neutralisation involving hydroxide ions (1 mark)
Reaction with a carbonate produces a carboxylate salt, carbon dioxide, and water OR mentions effervescence due to CO₂ (1 mark)
Maximum 5 marks

