OCR Specification focus:
‘Esterify carboxylic acids with alcohols using acid catalysts; anhydrides esterify alcohols readily.’
Introduction
Ester formation is a key synthetic process in organic chemistry, allowing efficient preparation of esters from carboxylic acids or acid anhydrides, often using acid catalysis.
Making Esters from Carboxylic Acids and Alcohols
Esterification between a carboxylic acid and an alcohol is a fundamental reaction in organic synthesis. This transformation is typically achieved through acid catalysis, most commonly using concentrated sulfuric acid (H₂SO₄), which increases the reaction rate and helps shift the equilibrium towards ester formation.
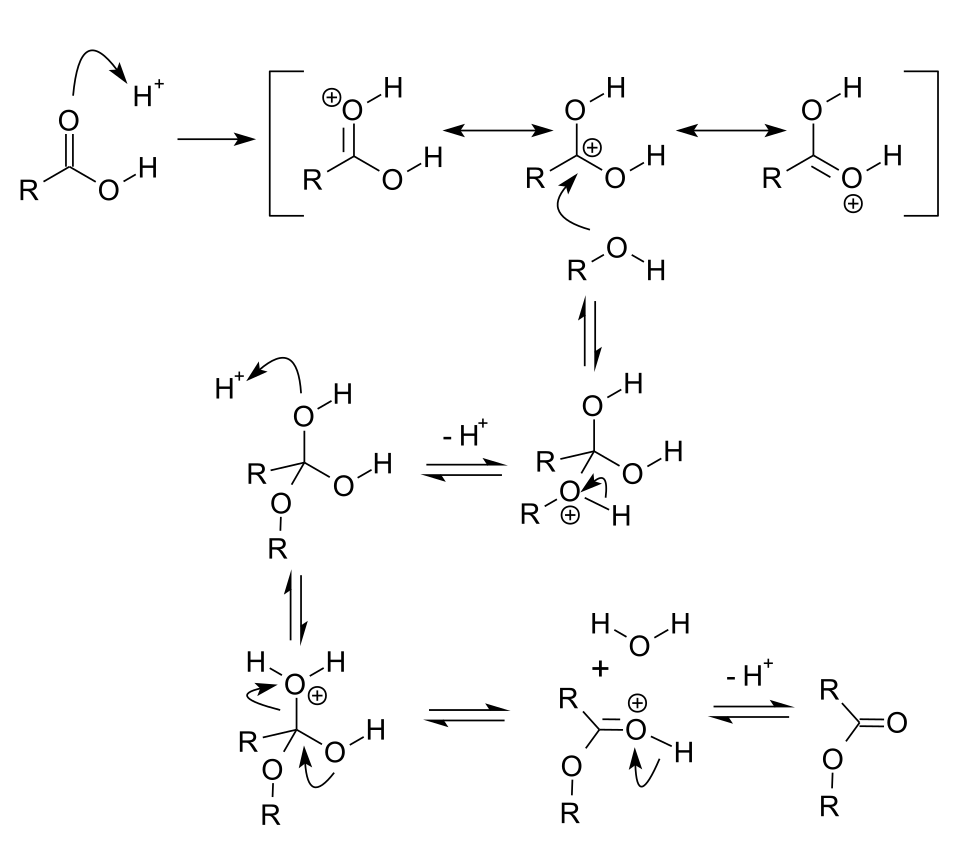
This scheme shows the Fischer esterification mechanism under acid catalysis, highlighting carbonyl activation, alcohol attack, and dehydration to form an ester. The catalyst is regenerated after proton transfers. Source
Key Features of Acid-Catalysed Esterification
Involves reaction of a carboxylic acid with an alcohol.
Produces an ester and water.
Requires an acid catalyst, which protonates the carboxyl group to enhance electrophilicity.
Is a reversible reaction and therefore does not always go to completion.
Reaction rate and yield depend on factors such as temperature, catalyst amount, and removal of water.
When the term acid catalyst is first introduced, it needs clear definition.
Acid catalyst: A substance that increases the rate of a reaction by donating protons but is chemically unchanged at the end.
After protonation of the carbonyl oxygen, the carboxylic acid becomes more susceptible to nucleophilic attack by the alcohol.
Mechanistic Outline (A-Level Scope)
Although a full mechanism is not always required, OCR expects students to understand the broad sequence of steps.
Protonation of the carbonyl oxygen in the carboxylic acid.
Nucleophilic attack by the alcohol on the activated carbonyl.
Proton transfers and loss of water.
Deprotonation of the ester to regenerate the catalyst.
A simple relationship describing equilibrium for esterification is useful.
Equilibrium Expression (Kc) = [Ester][Water] / [Carboxylic Acid][Alcohol]
Kc = Equilibrium constant (no units)
The position of equilibrium determines the proportion of ester formed.
Factors Affecting Yield
To improve ester yield in reversible esterification:
Use excess alcohol to shift equilibrium towards ester.
Distil off the ester as it forms if volatile.
Increase reaction temperature to improve reaction rate (up to safe limits).
Use strong acids such as H₂SO₄ to maximise catalytic efficiency.
Characteristics of Esters Formed from Carboxylic Acids
Many esters have distinctive fruity odours, making this reaction industrially important.
Esters generally exhibit lower boiling points than corresponding acids due to weaker intermolecular forces.
They are relatively non-polar, giving them good solvent properties.
These properties stem from the structural changes during ester formation.
Making Esters from Acid Anhydrides
Acid anhydrides are more reactive acyl derivatives than carboxylic acids, so they form esters more readily without requiring a strong acid catalyst. OCR emphasises that anhydrides esterify alcohols readily, and this reactivity difference is a significant syllabus focus.
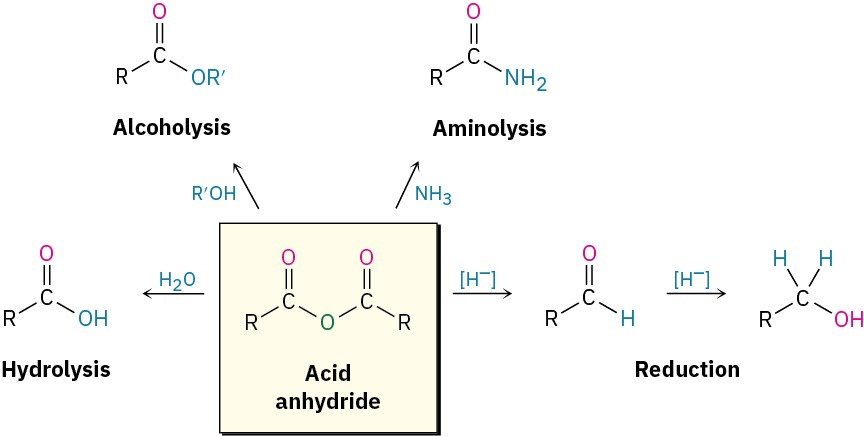
This diagram summarises reactions of acid anhydrides, including alcoholysis to form an ester. Other pathways shown extend beyond OCR requirements for this subsubtopic. Source
Why Anhydrides Are More Reactive
The anhydride functional group contains two acyl groups linked by an oxygen atom.
The carbonyl carbon in an anhydride is more electrophilic than in a carboxylic acid.
The reaction produces a carboxylic acid as a by-product rather than water.
The reaction is not reversible in the same sense as acid–alcohol esterification, allowing higher yields.
Features of Esterification Using Anhydrides
No strong acid catalyst is required, although mild heating may be used.
Reaction proceeds rapidly at room temperature or with gentle warming.
Useful for sensitive alcohols that may not withstand strongly acidic conditions.
Produces high purity esters with fewer side reactions.
A definition is appropriate when introducing acid anhydride.
Acid anhydride: An organic compound formed from two carboxylic acids by removal of water, containing two acyl groups bonded to the same oxygen.
This structure explains why anhydrides behave as strong electrophiles.
Reaction Steps for Alcohol + Acid Anhydride
Alcohol attacks the electrophilic carbonyl carbon of the anhydride.
Tetrahedral intermediate collapses, releasing a carboxylate group.
Proton transfer forms the ester and a carboxylic acid by-product.
A sentence is required before introducing an associated equation relating to this reaction type.
General Reaction (Ester Formation) = Alcohol + Acid Anhydride → Ester + Carboxylic Acid
Alcohol = Organic molecule containing a hydroxyl group (–OH)
Acid Anhydride = Reactive acyl compound derived from carboxylic acids
This form is widely used in synthesis because it minimises equilibrium limitations.
Comparing Acid-Catalysed and Anhydride Esterification
Understanding the differences helps students rationalise which reagent is more suitable.
Carboxylic Acids + Alcohols
Require acid catalyst.
Reversible reaction.
Slower and lower yielding.
Suitable for less reactive alcohols.
Acid Anhydrides + Alcohols
No catalyst needed.
Faster, higher-yielding process.
Produces a carboxylic acid, not water.
Preferred for laboratory ester preparation.
These distinctions are central to OCR’s emphasis on the ease with which anhydrides esterify alcohols.
FAQ
Concentrated sulfuric acid is preferred because it performs two useful roles simultaneously.
It provides H⁺ ions to catalyse the reaction by increasing the electrophilicity of the carbonyl carbon.
It also acts as a dehydrating agent, helping to remove water from the reaction mixture, which shifts the equilibrium towards ester formation.
Esterification between a carboxylic acid and an alcohol is an equilibrium reaction.
As water accumulates, it encourages the reverse reaction, converting the ester back into the original acid and alcohol.
This limits the maximum yield unless steps are taken to shift the equilibrium position.
Acid anhydrides contain two acyl groups bonded to the same oxygen, making their carbonyl carbons highly electrophilic.
This increased electrophilicity allows alcohols to act as nucleophiles without prior protonation.
As a result, ester formation occurs readily under mild conditions.
In reactions involving acid anhydrides, one acyl group is transferred to the alcohol to form the ester.
The remaining acyl group forms a carboxylic acid instead of water.
This difference explains why these reactions are not equilibrium-limited in the same way as acid–alcohol esterification.
Acid anhydrides are often chosen because they give higher yields and cleaner products.
The reaction is faster.
No strong acid catalyst is required.
Fewer equilibrium limitations occur.
These features make purification simpler and more reliable for laboratory-scale synthesis.
Practice Questions
A student reacts ethanol with ethanoic acid using concentrated sulfuric acid.
(a) State the role of concentrated sulfuric acid in this reaction.
(b) Name the organic product formed.
(2 marks)
(a)
Acts as an acid catalyst / provides H+ ions (1 mark)
(b)
Ethyl ethanoate (1 mark)
Ethanol can be used to form esters by reacting it with either ethanoic acid or ethanoic anhydride.
(a) Describe how ethanol reacts with ethanoic acid to form an ester, including the conditions required.
(b) Explain one advantage of using ethanoic anhydride instead of ethanoic acid to prepare the ester.
(5 marks)
(a) Description of esterification with ethanoic acid (up to 3 marks):
Ethanoic acid reacts with ethanol to form an ester and water (1 mark)
Reaction is acid-catalysed, typically using concentrated sulfuric acid (1 mark)
Reaction is reversible / equilibrium reaction / heated under reflux (1 mark)
(b) Advantage of using ethanoic anhydride (2 marks):
Reaction is faster or more vigorous (1 mark)
Reaction is not reversible / gives a higher yield of ester / does not produce water (1 mark)
Maximum 5 marks.

