OCR Specification focus:
‘Form acyl chlorides from carboxylic acids using SOCl₂ as the chlorinating reagent.’
Acyl chlorides are highly reactive derivatives formed from carboxylic acids. Their preparation is essential in synthetic chemistry, enabling efficient formation of esters, amides, and other functional groups.
Preparing Acyl Chlorides from Carboxylic Acids
The transformation of a carboxylic acid into an acyl chloride is a key reaction within organic synthesis and appears frequently in both industrial and laboratory contexts. OCR A-Level Chemistry emphasises the use of thionyl chloride (SOCl₂) as the standard reagent for this process. This method is preferred because it is efficient, produces few by-products, and drives the reaction to completion.
Key Features of Acyl Chlorides
Acyl chlorides are characterised by the functional group –COCl and exhibit significantly enhanced reactivity compared to the carboxylic acids from which they are derived. Their electrophilic carbonyl carbon makes them highly effective in condensation reactions with alcohols, phenols, and amines. They are moisture-sensitive, reacting vigorously with water.
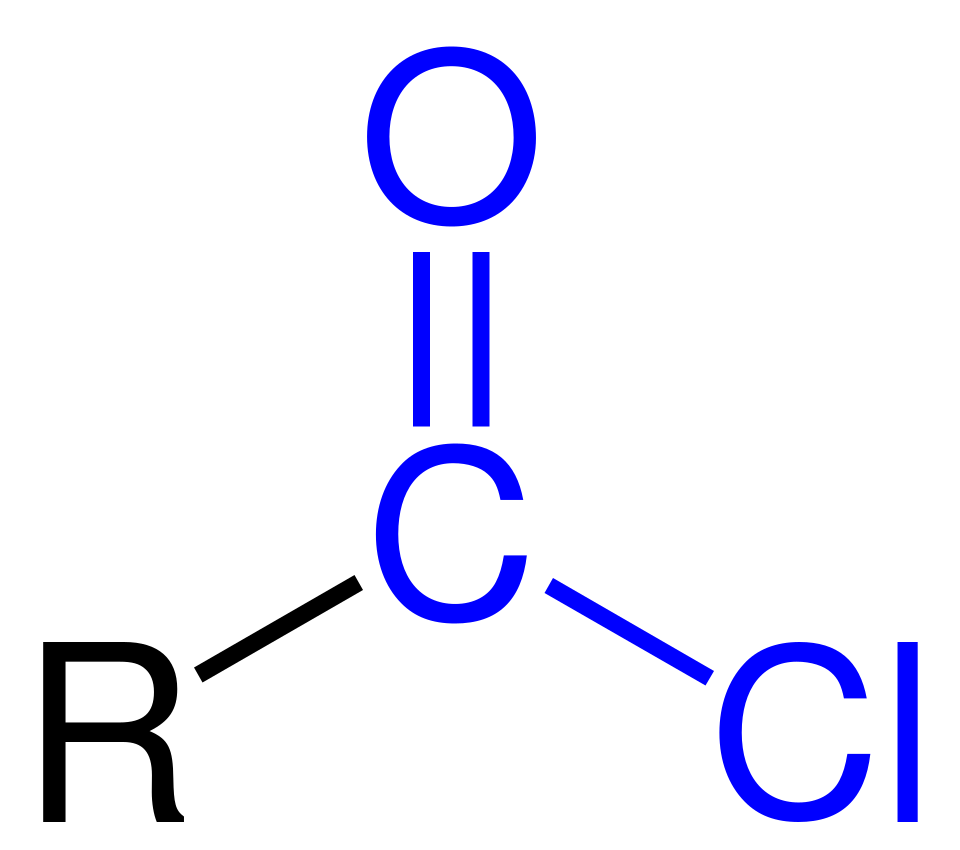
This diagram shows the general structural formula of an acyl chloride, R–C(=O)Cl. The carbonyl group and the chlorine atom attached to the acyl carbon define the –COCl functional group. Source
Reactivity of Carboxylic Acids with SOCl₂
Carboxylic acids themselves show limited reactivity because of strong hydrogen bonding and resonance stabilisation. The conversion to acyl chlorides removes the hydroxyl group and substitutes a chlorine atom, which significantly increases the susceptibility of the carbonyl carbon to further nucleophilic attack.
The reaction requires SOCl₂, usually used in excess.
The process is typically carried out under anhydrous conditions to prevent hydrolysis.
The reaction mixture often releases gaseous by-products, aiding purification of the acyl chloride.
A useful feature is that the by-products — sulfur dioxide (SO₂) and hydrogen chloride (HCl) — escape as gases, ensuring the forward reaction remains favourable.

This reaction scheme illustrates the conversion of a carboxylic acid to an acyl chloride using thionyl chloride. The formation of sulfur dioxide and hydrogen chloride gases helps drive the reaction to completion. Source
The Role of Thionyl Chloride (SOCl₂)
Thionyl chloride is the reagent specified by OCR for preparing acyl chlorides. It acts as both a chlorinating agent and a dehydrating agent, enabling the replacement of the –OH group in the carboxylic acid.
Chlorinating agent: A substance capable of introducing a chlorine atom into an organic compound by substitution.
SOCl₂ is preferred over reagents such as phosphorus pentachloride (PCl₅) or phosphorus trichloride (PCl₃) due to its cleaner gaseous by-products and ease of handling in synthesis.
A sentence placed here ensures spacing between blocks and maintains clarity before introducing the chemical equation.
Formation of Acyl Chlorides
RCOOH + SOCl₂ → RCOCl + SO₂ + HCl
RCOOH = Carboxylic acid (mol)
SOCl₂ = Thionyl chloride (mol)
RCOCl = Acyl chloride (mol)
SO₂ = Sulfur dioxide gas
HCl = Hydrogen chloride gas
Mechanistic Overview (Simplified for OCR)
Although a full mechanism is not required at A-Level, an outline helps to explain why SOCl₂ is so effective:
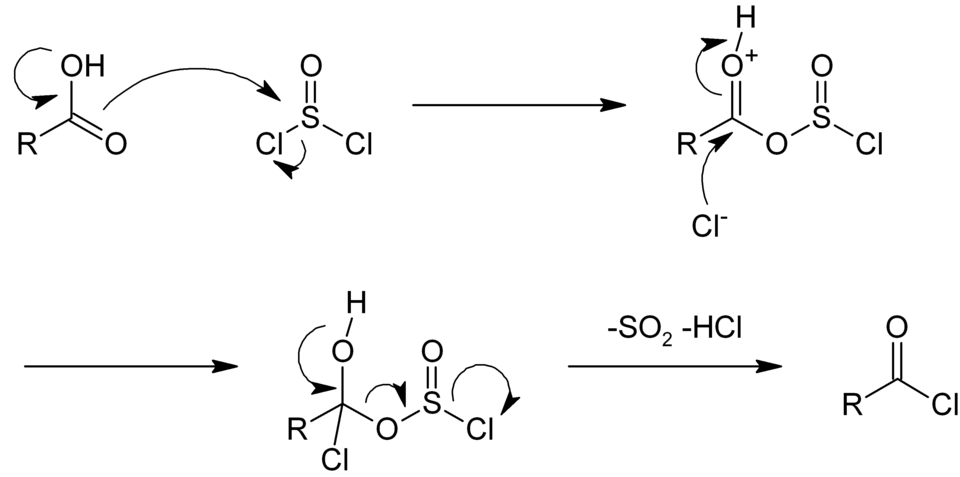
This diagram outlines the multi-step pathway by which thionyl chloride converts a carboxylic acid into an acyl chloride. Several intermediates and mechanistic details shown exceed OCR requirements but support the simplified explanation given in the notes. Source
Initial attack of the acid’s oxygen on the sulfur atom of SOCl₂.
Formation of an unstable intermediate containing both chlorine and –OSOCl groups.
Elimination of SO₂ and Cl⁻.
Final substitution step producing the acyl chloride and releasing HCl.
This layered sequence supports understanding of the reagent’s dual role.
Conditions Required
While the reaction typically proceeds at room temperature, specific features enhance safety and yield:
Anhydrous conditions: Prevents premature hydrolysis of the acyl chloride.
Fume cupboard: Necessary due to release of toxic SO₂ and HCl gases.
Dry glassware: Moisture severely compromises purity.
No need for a catalyst: SOCl₂ reacts directly and rapidly.
Why SOCl₂ Is Favoured
The reaction’s efficiency is linked to thermodynamic and practical considerations. Removal of gaseous products shifts equilibrium towards product formation. Additionally, acyl chlorides formed using SOCl₂ often require minimal purification, simplifying laboratory procedures.
Further reasons for its preference include:
High atom economy regarding the desired functional transformation.
Mild reaction conditions that do not damage sensitive functional groups.
Straightforward isolation of acyl chlorides without complex extraction steps.
Recognition and Behaviour of Acyl Chlorides Post-Preparation
Once prepared, acyl chlorides exhibit distinct properties:
They react with water to form carboxylic acids rapidly.
Their reactions are typically exothermic and produce visible fumes of HCl.
They are used immediately or stored under dry inert atmospheres.
These considerations matter for handling and safe laboratory practice.
Common Applications of Prepared Acyl Chlorides
Although full synthesis routes are discussed elsewhere, understanding how the prepared acyl chlorides are used provides context for their significance:
Ester formation: React with alcohols or phenols without the need for catalysts.
Amide formation: React with ammonia or amines, giving high yields.
Production of acid anhydrides: The acyl chloride reacts with carboxylate ions.
This highlights why their preparation is embedded in many organic transformation pathways.
Practical Considerations and Safety
Handling SOCl₂ and acyl chlorides requires caution:
SOCl₂ is corrosive and releases harmful vapours when exposed to moisture.
Reactions must occur in a fume cupboard to prevent inhalation of toxic gases.
Acyl chlorides must be stored in sealed, dry containers to prevent degradation.
Summary of Key Points for OCR
SOCl₂ is the required reagent for converting carboxylic acids to acyl chlorides.
The reaction produces SO₂ and HCl gases, which remove themselves from the mixture.
Reaction conditions must be dry, controlled, and well-ventilated.
The resulting acyl chlorides serve as reactive intermediates in further synthesis.
These notes provide essential specification-aligned understanding of preparing acyl chlorides from carboxylic acids using SOCl₂.
FAQ
Thionyl chloride is highly effective because it reacts directly with the –OH group of the carboxylic acid and forms unstable intermediates that rapidly decompose.
The key advantage is that both by-products are gases, which leave the reaction mixture immediately. This makes the reaction effectively irreversible and efficient under mild conditions.
Water reacts rapidly with both thionyl chloride and the acyl chloride product.
This leads to:
Hydrolysis of the acyl chloride back to the carboxylic acid
Reduced yield of the desired product
Release of hydrogen chloride gas
For this reason, dry conditions and apparatus are essential.
Acyl chlorides are highly reactive and unstable in the presence of moisture in the air.
Over time, they react with trace amounts of water to form the original carboxylic acid and hydrogen chloride. This makes long-term storage impractical unless completely dry, airtight conditions are used.
The chlorine atom attached to the carbonyl carbon is strongly electron-withdrawing.
This increases the partial positive charge on the carbonyl carbon, making it very susceptible to nucleophilic attack. As a result, acyl chlorides are much more reactive than carboxylic acids in substitution reactions.
Thionyl chloride reacts violently with water and produces toxic gases.
Specific hazards include:
Release of sulfur dioxide, which is toxic and irritating
Formation of hydrogen chloride gas, which is corrosive
Strong reactivity with moist air and skin
These hazards require strict use of a fume cupboard and appropriate protective equipment.
Practice Questions
A student prepares an acyl chloride from a carboxylic acid using thionyl chloride.
a) Name the reagent used to convert a carboxylic acid into an acyl chloride.
b) State one observation that would be seen during this reaction.
(2 marks)
a) Thionyl chloride or SOCl₂ (1 mark)
b) Any one of the following (1 mark):
Fumes of hydrogen chloride
Gas produced / effervescence
Sulfur dioxide gas released
Ethanoic acid can be converted into ethanoyl chloride using thionyl chloride, SOCl₂.
a) Write a balanced chemical equation for the reaction between ethanoic acid and thionyl chloride.
b) Explain why this reaction goes to completion.
c) State two safety precautions that should be taken when carrying out this reaction in the laboratory.
(5 marks)
a) Correct balanced equation (2 marks):
CH₃COOH + SOCl₂ → CH₃COCl + SO₂ + HCl
One mark for correct reactants and products
One mark for correct balancing
b) Explanation (1 mark):
Gaseous products (SO₂ and HCl) are removed from the reaction mixture
orRemoval of gases shifts equilibrium to the products
c) Safety precautions (2 marks):
Carry out the reaction in a fume cupboard
Avoid contact with skin / wear gloves
Avoid inhalation of gases
Use dry apparatus to prevent hydrolysis
Maximum 2 marks for two correct, distinct safety points.

