OCR Specification focus:
‘Hot aqueous acid gives acid and alcohol; hot aqueous alkali gives carboxylate salts and alcohol.’
Hydrolysis of Esters: Acid Versus Alkali
Ester hydrolysis breaks an ester into two products, and the reaction pathway depends strongly on whether hot aqueous acid or hot aqueous alkali is used.
Introduction
Ester hydrolysis involves breaking the ester functional group using water under acidic or alkaline conditions, producing different organic products depending on the reagents and reaction environment employed.
The Nature of Ester Hydrolysis
Esters contain the functional group –COO– linking an acyl group to an alcohol-derived alkoxy group. Hydrolysis reverses esterification by cleaving this linkage through reaction with water. Hydrolysis is a key process in organic synthesis, enabling conversion of esters into useful carboxylic acid derivatives or alcohols. The OCR specification distinguishes clearly between acid hydrolysis and alkali hydrolysis, each with different mechanistic features and outcomes.
Understanding Hydrolysis
Hydrolysis refers to the reaction in which a covalent bond is broken by water.
Hydrolysis: A reaction in which water breaks a covalent bond, forming two or more new molecules.
In ester hydrolysis, the carbonyl carbon is the key reactive site because its partial positive charge makes it vulnerable to attack by nucleophiles, whether water molecules in acidic conditions or hydroxide ions in alkaline conditions.
Acid Hydrolysis of Esters
Hot aqueous acid catalyses ester hydrolysis, acting as a reversible process that reforms the original carboxylic acid and alcohol. Strong acids such as HCl or H2SO4 are typically used as catalysts. The reaction is an equilibrium because the products can recombine to regenerate the ester.
Key Features of Acid Hydrolysis
Requires hot aqueous acid (usually dilute HCl or dilute H2SO4).
Produces a carboxylic acid and an alcohol.
Reaction is reversible and must be driven to completion by using excess water or removing products.
The acid acts as a catalyst, protonating the ester to increase electrophilicity at the carbonyl carbon.
Mechanistic Overview
Although full mechanisms are not required at this stage, it is important to understand that protonation of the ester carbonyl enhances its susceptibility to nucleophilic attack. Water acts as the nucleophile, attacking the carbonyl carbon and eventually generating the two products.
Hot aqueous acid hydrolyses an ester to a carboxylic acid and an alcohol, but the reaction is reversible and does not go to completion.
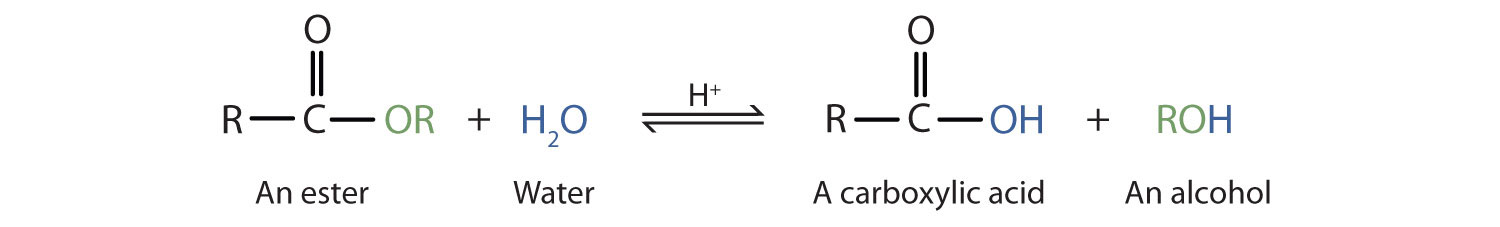
This diagram shows acid-catalysed hydrolysis of an ester producing a carboxylic acid and an alcohol. The equilibrium arrow highlights that, in acid, the reaction is reversible and forms a mixture rather than going to completion. Source
Alkali Hydrolysis of Esters (Saponification)
Under hot aqueous alkali conditions, ester hydrolysis is no longer reversible, making it a key synthetic route when complete conversion is desired. Hydroxide ions (OH–) act as strong nucleophiles, attacking the ester directly.
Key Features of Alkali Hydrolysis
Requires hot aqueous alkali, commonly NaOH or KOH.
Produces a carboxylate salt and an alcohol, matching the OCR specification.
Reaction is irreversible because the carboxylate anion is resistant to further reaction with alcohol.
This process is known as saponification when applied to triglycerides to produce soap.
Why Alkali Hydrolysis Is Irreversible
During the reaction, hydroxide ions attack the ester to form a tetrahedral intermediate that collapses, releasing an alcohol. The newly formed carboxylic acid is immediately deprotonated by excess hydroxide to give a carboxylate ion.
Carboxylate ion: The conjugate base of a carboxylic acid, formed by deprotonation of the –COOH group.
Because the carboxylate ion is negatively charged and not electrophilic enough to react with an alcohol, the reaction cannot reverse, ensuring complete hydrolysis.
Hot aqueous alkali hydrolyses an ester to a carboxylate salt and an alcohol; the reaction goes to completion.
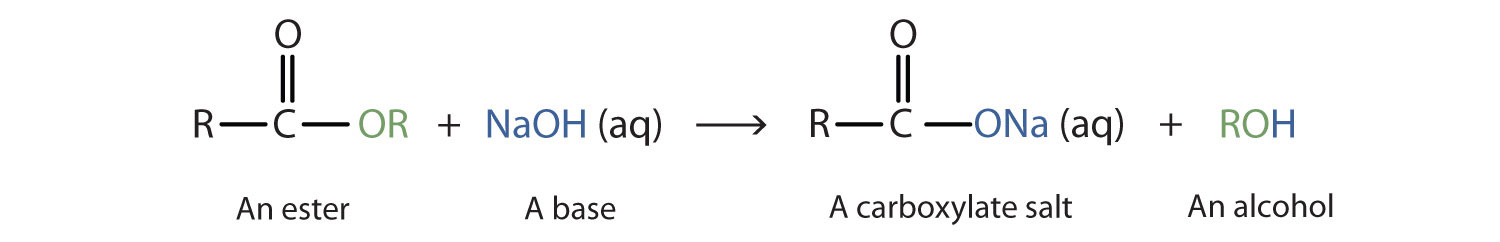
This diagram shows alkaline hydrolysis (saponification), where an ester reacts with aqueous hydroxide to form a carboxylate salt and an alcohol. The formation of an ionic carboxylate explains why the reaction is treated as going to completion. Source
Comparing Acid and Alkali Hydrolysis
Understanding the distinctions between these pathways is essential for predicting products and planning synthetic routes.
Product Differences
Acid hydrolysis: Produces a carboxylic acid and an alcohol.
Alkali hydrolysis: Produces a carboxylate salt and an alcohol, aligning precisely with the OCR specification.
Conditions
Acid hydrolysis requires hot aqueous acid in excess.
Alkali hydrolysis requires hot aqueous alkali, often used in stoichiometric excess.
Reversibility
Acid hydrolysis is reversible, forming an equilibrium mixture.
Alkali hydrolysis is irreversible, allowing quantitative conversion of an ester into its products.
General Acid Hydrolysis of an Ester = Ester + Water ⇌ Carboxylic Acid + Alcohol
Ester = RCOOR′ (R/R′ are alkyl groups)
Water = H2O
Carboxylic Acid = RCOOH
Alcohol = R′OH
This representation highlights the reversible nature of acid hydrolysis.
A sentence explaining the context ensures separation before the next equation block.
General Alkali Hydrolysis (Saponification) = Ester + OH– → Carboxylate Ion + Alcohol
OH– = Hydroxide ion, strong nucleophile
Carboxylate Ion = RCOO–, stable anion
Alcohol = R′OH
Practical Significance
These reactions are vital tools in organic synthesis and analytical chemistry. Acid hydrolysis is used when regenerating carboxylic acids is necessary, while alkali hydrolysis is chosen when complete, irreversible conversion is desired. The ability to control product formation makes ester hydrolysis an essential technique for A-Level chemists studying carbonyl derivative reactions.
In practice, ester hydrolysis is typically heated to keep the reaction mixture hot for an extended period.
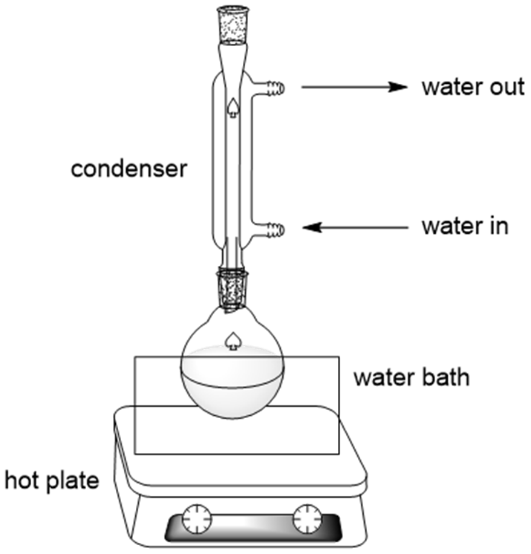
This diagram illustrates a standard reflux setup, allowing an aqueous reaction mixture to be heated continuously without loss of volatile components. The labelled condenser demonstrates how vapour condenses and returns to the reaction flask, supporting the use of hot aqueous conditions. Source
FAQ
Excess water shifts the equilibrium position towards hydrolysis by increasing the concentration of a reactant.
This reduces the likelihood of the reverse esterification reaction occurring and increases the yield of carboxylic acid and alcohol.
In laboratory practice, using a large volume of water is a simple way to favour hydrolysis without changing the catalyst.
Alkali hydrolysis produces a carboxylate salt, not a carboxylic acid.
To obtain the free carboxylic acid, the reaction mixture is acidified with a strong acid such as hydrochloric acid.
This protonates the carboxylate ion, converting it into the corresponding carboxylic acid.
Ester hydrolysis is a slow reaction at room temperature.
Heating increases the kinetic energy of reacting particles, leading to more frequent successful collisions.
Reflux allows prolonged heating without loss of volatile reactants or products, ensuring consistent reaction conditions.
Sodium hydroxide and potassium hydroxide both hydrolyse esters effectively.
The choice mainly affects the identity and solubility of the carboxylate salt formed.
Potassium carboxylates are often more soluble in water than sodium carboxylates, which can influence product handling.
Alkali hydrolysis is irreversible, allowing predictable and complete conversion of esters.
This reduces the need for equilibrium control and simplifies reaction optimisation.
As a result, alkali hydrolysis is commonly used where high yields and consistent product formation are required.
Practice Questions
An ester is heated under reflux with hot aqueous sodium hydroxide.
State the two organic products formed and explain why this reaction goes to completion.
(2 marks)
States formation of a carboxylate salt (or carboxylate ion) (1 mark)
States formation of an alcohol (1 mark)
OR, for explanation-based marking:
Explains that hydroxide ions produce a carboxylate ion/salt which cannot react back with alcohol (1 mark)
States that this makes the reaction irreversible or that it goes to completion (1 mark)
Maximum 2 marks.
A student carries out the hydrolysis of an ester using hot aqueous acid.
(a) Name the two organic products formed.
(b) State one strong acid that could be used in this reaction.
(c) Explain, in terms of reaction reversibility and product stability, why acid hydrolysis does not go to completion, whereas alkali hydrolysis does.
(5 marks)
(a)
Carboxylic acid identified (1 mark)
Alcohol identified (1 mark)
(b)
Correct acid stated, e.g. hydrochloric acid or sulfuric acid (1 mark)
(c)
States that acid hydrolysis is reversible / establishes an equilibrium (1 mark)
Explains that products can react together to reform the ester (1 mark)
States that alkali hydrolysis forms a carboxylate ion/salt (1 mark)
Explains that the carboxylate ion is stable or does not react with alcohol, so the reaction goes to completion (1 mark)
Maximum 5 marks.

