OCR Specification focus:
‘Polyamides form from acids or acyl chlorides with amines/diamines by condensation polymerisation.’
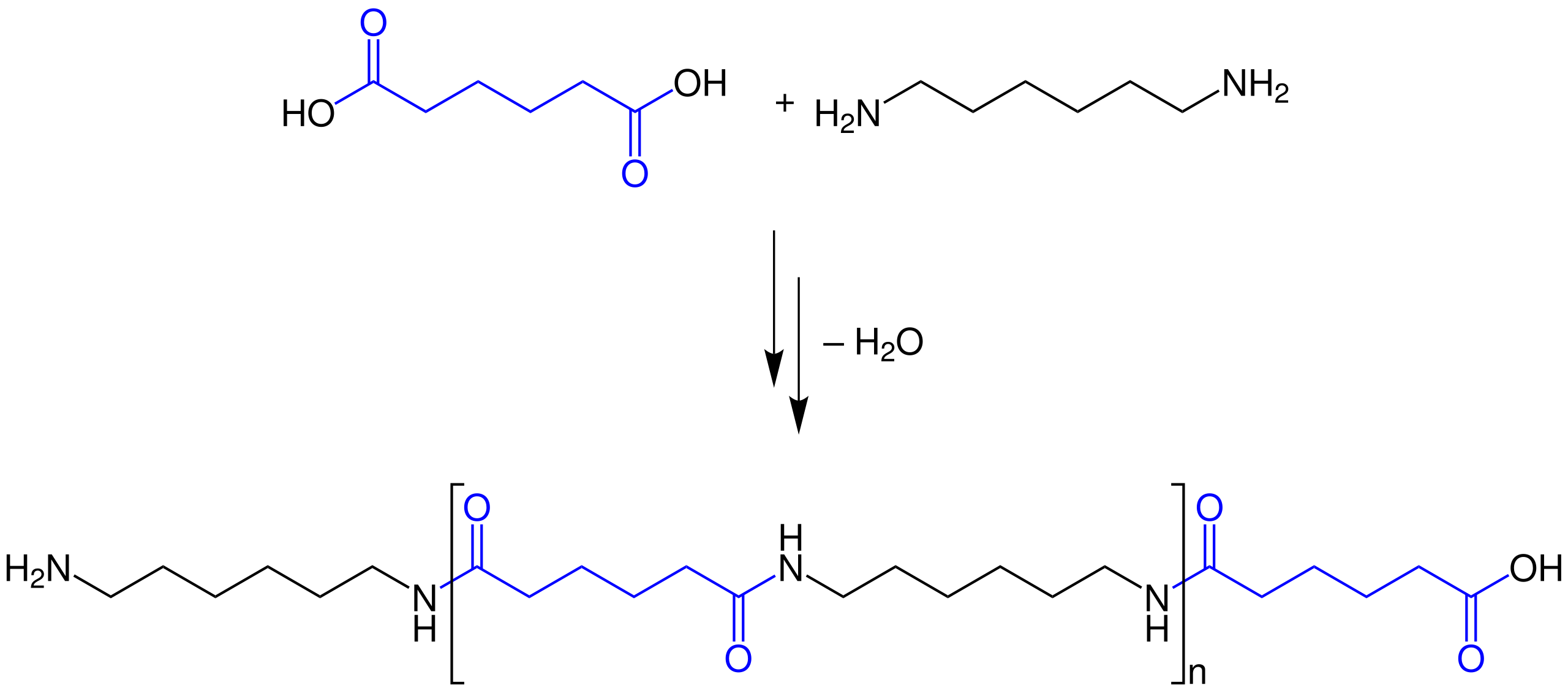
This diagram shows nylon‑6,6 forming via step‑growth condensation between a dicarboxylic acid and a diamine, creating repeating amide (–CONH–) links. Each new link forms with elimination of water, so the polymer grows by repeated condensation. Source
Polyamides form through condensation polymerisation, linking monomers with amide bonds and releasing small molecules. This subsubtopic explains the formation of polyamides from suitable monomers.
Making Polyamides by Condensation
Polyamides are an important class of condensation polymers created when carboxylic acids or acyl chlorides react with amines or diamines. The reaction forms an amide linkage and eliminates a small molecule, typically water or hydrogen chloride. Condensation polymerisation generates long chains using monomers with two functional groups, ensuring the chain can extend indefinitely. Understanding these structural and mechanistic principles is essential for analysing polymer formation pathways in A‑Level Chemistry.
Key Functional Groups Involved
Polyamide formation relies on two complementary functional groups — carboxyl groups (–COOH or –COCl) and amine groups (–NH₂). Their reactivity determines both the feasibility and efficiency of polymerisation.
Amide linkage: The functional group –CONH– formed when a carboxyl group reacts with an amine group.
When a carboxyl group reacts with an amine group, a condensation reaction occurs, producing an amide link and releasing a small molecule. This small‑molecule loss is what distinguishes condensation polymerisation from addition polymerisation.
Types of Monomers Used
Polyamides can be formed from different combinations of monomers depending on the desired polymer structure and properties.
Dicarboxylic acid + diamine
Produces a chain in which each monomer unit contributes an amide linkage.
Water is released at each step of the polymerisation.
Diacyl chloride + diamine
Forms polyamides more readily due to the higher reactivity of acyl chlorides.
Hydrogen chloride is released rather than water.
Amino acid monomers (containing both –COOH and –NH₂ in the same molecule)
Can form polyamides without the need for separate monomers.
Each amino acid contributes both functional groups to polymer growth.
The requirement for bifunctional monomers ensures continual chain extension, as each reactive end allows further condensation to occur.
Condensation Polymerisation Process
The process of forming polyamides involves repeated formation of amide linkages. The steps below outline the general pattern of condensation polymerisation:
Alignment of monomers so that carboxyl and amine groups approach each other.
Nucleophilic attack by the amine nitrogen on the carbonyl carbon of the acid or acyl chloride.
Formation of the amide linkage and simultaneous elimination of water or hydrogen chloride.
Propagation as each new reactive end continues to react with additional monomers.
Because each monomer contains two reactive groups, alternating condensations lead to long‑chain polymer structures.
Role of Acyl Chlorides in Polyamide Formation
Acyl chlorides are significantly more reactive than carboxylic acids. Their carbonyl carbon is strongly electrophilic, enabling faster nucleophilic attack by amines. This often makes acyl chlorides the preferred monomers for laboratory preparation of polyamides.
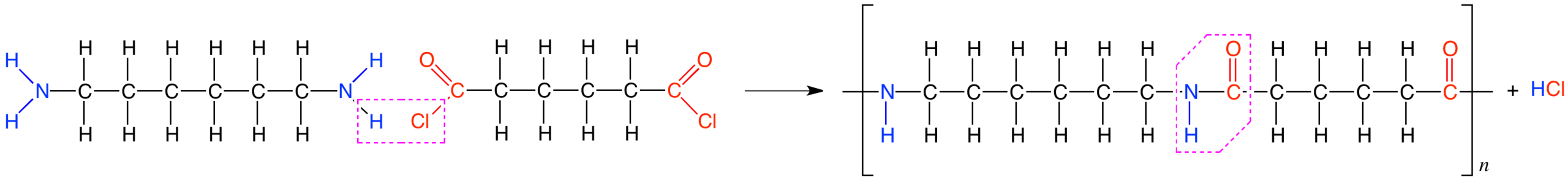
This scheme shows nylon‑6,6 forming when a diacyl chloride reacts with a diamine, creating repeating amide links along the chain. The by‑product is hydrogen chloride rather than water, reflecting the higher reactivity of acyl chlorides. Source
Condensation polymerisation: A polymer‑forming process in which monomers join together with the elimination of small molecules such as water or hydrogen chloride.
Acyl chlorides react vigorously with amines at room temperature, simplifying the production of polyamides under mild conditions and reducing the need for heating. However, hydrogen chloride produced during the reaction can protonate free amines, so a base is often added to neutralise the acid and maintain polymer growth.
Structural Features of Polyamides
Polyamides contain repeating amide linkages, which introduce strong hydrogen bonding between polymer chains. This intermolecular attraction gives polyamides high melting points, mechanical strength, and durability, making them valuable materials in both industrial and consumer applications.
The –CONH– group encourages linear chain alignment.
Strong hydrogen bonding leads to crystalline regions within the polymer.
Polyamides exhibit resistance to stretching and significant tensile strength.
Their chemical resistance varies depending on side‑chain substituents and monomer spacing.
These physical characteristics arise directly from the amide linkage’s polarity and ability to form intermolecular interactions.
Example Formation Pathways (Conceptual Only)
While no worked examples or calculations are included, it is useful to understand how the general reactions map onto polyamide formation:
A diamine reacting with a dicarboxylic acid forms a repeating –NH–CO– pattern throughout the chain.
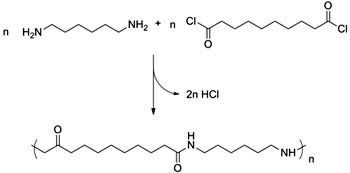
A diamine reacting with a diacyl chloride follows the same structural pattern but produces HCl rather than water.
Amino acids undergoing self‑condensation create polyamides with one amide bond per monomer unit.
Each of these pathways aligns with the specification requirement that polyamides form from acids or acyl chlorides with amines/diamines.
Importance in Synthetic Pathways
Polyamide synthesis is a key part of designing extended carbon‑based structures, linking directly to broader organic chemistry topics such as functional group interconversion and polymer design. Understanding their formation allows prediction of polymer repeat units, assessment of monomer suitability, and the ability to propose synthetic routes.
This photograph shows the nylon rope trick, where a polyamide film forms at the interface between two immiscible solutions and can be pulled out as a continuous strand. It demonstrates rapid condensation polymerisation at a liquid–liquid boundary; the use of a power drill mentioned on the page is beyond syllabus requirements. Source
Overall, the essential idea is that polyamides arise from condensation between complementary functional groups, creating long‑chain molecules characterised by amide linkages and the elimination of small molecules at every step.
FAQ
Polyamides contain amide (–CONH–) groups, which are highly polar. This allows strong hydrogen bonding to form between neighbouring polymer chains.
These intermolecular attractions require significant energy to overcome, resulting in high melting points and good thermal stability compared with many addition polymers.
When diacyl chlorides react with diamines, hydrogen chloride is produced.
If not removed, HCl can protonate amine groups, preventing further polymer growth. A base is added to:
Neutralise hydrogen chloride
Maintain a supply of unprotonated amine groups
Allow the condensation polymerisation to continue efficiently
Condensation polymerisation is a step-growth process rather than a chain-growth process.
Polymer chains grow gradually as monomers and short chains react randomly. This leads to a mixture of chain lengths rather than uniform polymers, especially if monomers are not present in exact stoichiometric ratios.
The distance between amide linkages depends on the length of the monomers used.
Shorter spacings increase hydrogen bonding density, leading to:
Higher melting points
Increased rigidity
Longer spacings reduce intermolecular attraction, producing more flexible polyamides.
Water is a product of condensation between carboxylic acids and amines.
If water accumulates, it can drive the equilibrium backwards and limit polymer formation. Removing water:
Shifts equilibrium towards polymer formation
Encourages longer polymer chains to form
Improves overall yield of the polyamide
Practice Questions
Describe how a polyamide is formed by condensation polymerisation from a dicarboxylic acid and a diamine.
(2 marks)
Formation of amide linkages between the monomers. (1 mark)
Elimination of water as the small molecule during condensation polymerisation. (1 mark)
A polyamide is made by reacting a diamine with a diacyl chloride.
(a) Explain why diacyl chlorides are more reactive than dicarboxylic acids in polyamide formation.
(b) Describe the type of bond formed between monomer units and name the small molecule eliminated during this polymerisation.
(c) Explain why monomers used to make polyamides must contain two functional groups.
(5 marks)
(a)
Diacyl chlorides are more reactive because the carbonyl carbon is more electrophilic / the C–Cl bond is easily broken. (1 mark)
(b)
Formation of an amide (–CONH–) bond between monomer units. (1 mark)
Hydrogen chloride (HCl) is eliminated as the small molecule. (1 mark)
(c)
Each monomer must have two functional groups so the polymer chain can continue to grow. (1 mark)
Allows formation of long chains rather than small molecules. (1 mark)

