OCR Specification focus:
‘Given monomer(s), predict the polymer repeat unit and polymerisation type.’
Predicting repeat units from monomers is a key skill in polymer chemistry, enabling students to connect monomer structures to the backbone pattern formed during polymerisation. This topic supports understanding of how functional groups determine polymer type and how atoms rearrange as small molecules are eliminated or as double bonds open during polymer formation.
Understanding Repeat Units in Polymer Chemistry
A repeat unit is the smallest structural segment that repeats continuously along the polymer chain. It reflects the atoms that remain after the polymerisation reaction has occurred. Students should be able to identify the repeat unit directly from monomer structures and determine whether the process is addition polymerisation or condensation polymerisation.
Polymerisation type depends on the monomers’ functional groups: alkenes typically undergo addition polymerisation, while molecules containing carboxylic acids, alcohols, amines, acyl chlorides, or diols/diamines typically undergo condensation polymerisation because small molecules such as H₂O or HCl are eliminated.
Repeat Unit: The smallest continuous structural fragment of a polymer that represents the atoms incorporated from monomers after polymerisation.
To identify a repeat unit, students must recognise how the monomer functional group reacts and what structural changes occur when the polymer backbone forms.
Addition Polymerisation and Predicting Repeat Units
Key features of addition polymerisation
Addition polymerisation occurs when monomers with C=C double bonds join together, with no atoms lost in the overall reaction. The double bond opens and links monomers into a continuous chain.
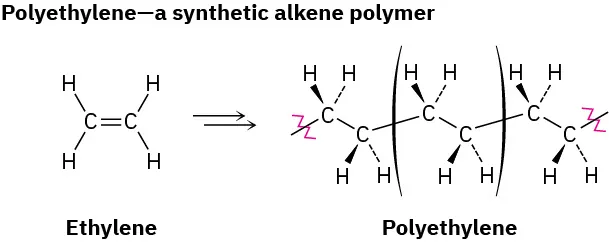
This diagram shows ethene monomers polymerising into poly(ethene) as the C=C double bond opens to form a C–C single-bond backbone. The bracketed segment represents the repeat unit, and n indicates many repeats along the chain. Source
The resulting repeat unit contains the carbon chain from the monomer, now with single bonds.
Identifying repeat units from alkene monomers
When predicting an addition polymer repeat unit:
Identify the alkene functional group.
Convert the C=C double bond into a C–C single bond in the polymer backbone.
Ensure substituents attached to the original alkene are shown in the same relative positions.
Remove any polymer end groups; only the repeating core should appear.
Use bracketed repeat-unit notation if required by diagrams (though not reproduced here in text).
These principles allow students to see that the repeat unit corresponds exactly to the monomer minus the double bond.
A normal sentence is placed here to maintain the required spacing before introducing the next definition.
Addition Polymerisation: A polymer-forming reaction where unsaturated monomers join without the loss of small molecules.
Condensation Polymerisation and Repeat Unit Prediction
Condensation polymerisation involves monomers with two functional groups. Each end reacts with another monomer, forming chains while eliminating a small molecule such as H₂O or HCl. The repeat unit therefore differs from the monomer(s) because certain atoms are removed during the condensation process.
Typical monomer pairs in condensation polymerisation
Polyesters and polyamides, both emphasised in this part of the specification, form from specific functional-group combinations. When determining the repeat unit, students should consider:
Diacid + diol → polyester
Diacyl chloride + diol → polyester
Diacid + diamine → polyamide
Diacyl chloride + diamine → polyamide
Structural changes that determine repeat units
Predicting a condensation polymer repeat unit requires identifying which atoms are removed during formation of the ester linkage or amide linkage.
For polyesters:
The carboxyl group of the acid or acyl chloride loses an –OH (or –Cl).
The alcohol or diol loses a hydrogen from its –OH group.
The resulting linkage is –COO–.
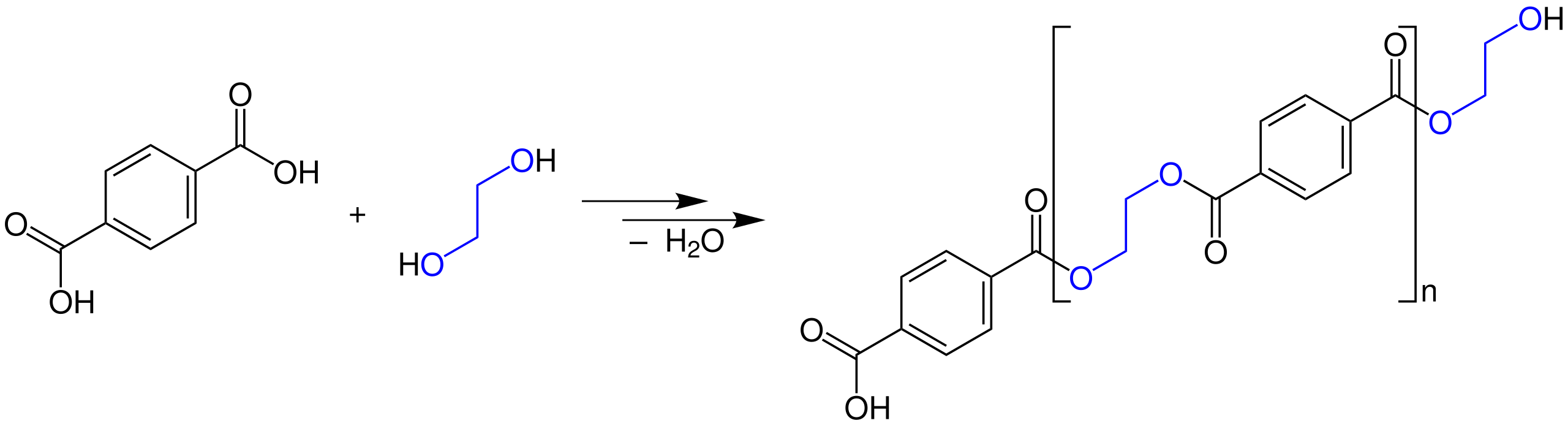
This diagram shows a diol reacting with a dicarboxylic acid (or derivative) to form a polyester, highlighting the repeated ester link (–COO–) in the chain. The bracketed section indicates the repeat unit that is copied many times along the polymer backbone. Source
For polyamides:
The carboxyl group loses an –OH (or –Cl from an acyl chloride).
The amine or diamine loses a hydrogen from –NH₂.
The resulting linkage is –CONH–.
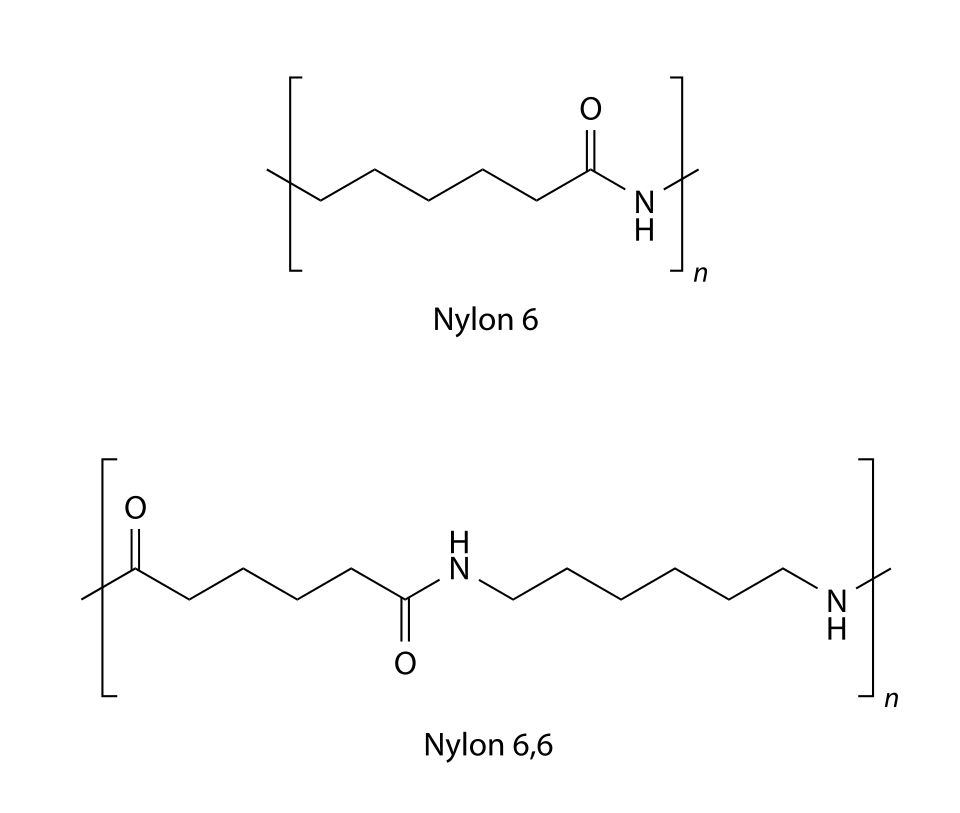
This figure shows polymer-chain segments for nylon (polyamides), making the repeating amide link (–CONH–) easy to spot. It also compares two nylon types, which goes slightly beyond the syllabus, but the key feature is the shared amide linkage within the repeat unit. Source
Because the repeat unit must show the final linkage, students must mentally remove eliminated atoms and redraw the connecting groups accordingly.
A normal sentence is placed here before the next definition block.
Condensation Polymerisation: A polymer-forming reaction in which monomers join with the simultaneous elimination of a small molecule such as water or hydrogen chloride.
Step-by-Step Method for Predicting Repeat Units
1. Identify the functional groups in each monomer
Understanding the reactive groups determines whether the process is addition or condensation. Functional groups such as carboxylic acids, acyl chlorides, alcohols, and amines directly influence the type of polymer linkage.
2. Determine which atoms will be retained and which will be eliminated
For addition reactions, no atoms are lost.
For condensation reactions, compute the atoms removed from the functional groups forming the new linkage.
3. Draw the backbone connection formed after polymerisation
For addition polymers: draw the former double-bond carbons now joined by a single bond.
For condensation polymers: draw the bond between the carbonyl carbon and the oxygen or nitrogen, depending on whether the polymer is a polyester or polyamide.
4. Remove monomer end groups that do not appear in the repeat unit
Because repeat units represent internal segments of the polymer chain, terminal hydrogens or functional groups that would appear only on chain ends must be excluded.
5. Ensure the repeat unit accurately reflects the final polymer structure
Key checks include:
Substituents must remain attached in the correct positions.
The order of atoms must reflect the directionality of the monomer connection.
Only atoms present in the polymer chain after polymer formation should be shown.
Determining Polymerisation Type from Monomers
Students must also be able to state whether the polymerisation mechanism is addition or condensation, based solely on monomer structure. The identification depends on:
Presence of an alkene, indicating addition polymerisation.
Presence of two functional groups capable of reacting together (e.g. –COOH and –OH), indicating condensation polymerisation.
Whether any small molecule would logically be eliminated.
This skill directly links to multistep synthesis contexts, where polymers can serve as targets or intermediates.
Importance of Predicting Repeat Units in Organic Synthesis
Understanding how monomers convert to polymer backbones enhances knowledge across organic chemistry, including mechanisms, functional group reactivity, and synthetic transformations. It also consolidates concepts from carboxylic acids, alcohols, amines, acyl chlorides, and nitriles, supporting broader specification themes.
FAQ
The orientation of a repeat unit is not unique, but it must be chemically correct. You can draw the repeat unit starting at any point along the polymer backbone, as long as the atom order and bonding are consistent.
What matters is that:
All bonds reflect the correct linkages formed during polymerisation
Functional groups are connected to the correct atoms
The structure would repeat seamlessly in both directions
Examiners accept equivalent repeat units drawn in different orientations if the connectivity is correct.
The repeat unit represents the structure of the polymer chain after polymerisation has occurred. Any small molecule eliminated, such as water or hydrogen chloride, is not part of the polymer backbone.
Including eliminated atoms would incorrectly suggest they remain in the polymer. Repeat units therefore show only:
The atoms retained in the chain
The newly formed ester or amide linkage
This ensures the repeat unit reflects the true structure of the polymer.
The final repeat unit is the same whether a carboxylic acid or an acyl chloride is used, because both form the same ester or amide linkage in the polymer.
The difference lies only in the eliminated molecule:
Carboxylic acid reactions eliminate water
Acyl chloride reactions eliminate hydrogen chloride
When predicting the repeat unit, you ignore the eliminated molecule and focus on the linkage formed in the polymer chain.
Frequent errors include:
Leaving the C=C double bond unchanged in addition polymers
Forgetting to remove –OH, –H, or –Cl groups in condensation polymers
Including end groups that would only appear at the ends of polymer chains
Drawing monomers rather than the polymer structure
Carefully identifying functional groups and considering which atoms remain after polymerisation helps avoid these mistakes.
In OCR A-Level Chemistry, polymers are usually treated as having a single repeat unit derived from one or two monomers. However, some real polymers are copolymers made from more than one monomer type.
For this specification:
You are only expected to predict a single repeat unit
Monomers are assumed to react in a regular, repeating pattern
More complex polymer structures are beyond the scope of this subsubtopic.
Practice Questions
Ethene is used to make poly(ethene).
a) State the type of polymerisation that ethene undergoes.
b) Explain why the repeat unit of poly(ethene) contains only single carbon–carbon bonds.
(2 marks)
a)
Addition polymerisation (1 mark)
b)
The C=C double bond in ethene opens during polymerisation (1 mark)
(Allow: double bond breaks and forms single bonds in the polymer chain)
A polymer is formed by reacting a diol with a dicarboxylic acid.
a) State the type of polymerisation involved.
b) Identify the functional groups present in the monomers.
c) Describe how the repeat unit of the polymer is formed from the monomers, referring to atoms removed during polymerisation.
(5 marks)
a)
Condensation polymerisation (1 mark)
b)
Alcohol (hydroxyl) functional group in the diol (1 mark)
Carboxylic acid functional group in the dicarboxylic acid (1 mark)
c)
The –OH from the carboxylic acid and an H from the alcohol are removed (1 mark)
This forms an ester linkage (–COO–) in the polymer repeat unit (1 mark)
(Maximum 5 marks)

