OCR Specification focus:
‘Polyesters form from acids or acyl chlorides with alcohols/diols by condensation polymerisation.’
Polyesters are an important class of synthetic polymers formed when monomers link through ester bonds, producing long chains and eliminating small molecules such as water or hydrogen chloride. This subsubtopic focuses on how condensation reactions produce polyester structures essential in fibres, plastics, and biochemical applications.
Making Polyesters by Condensation
Condensation polymerisation is a key synthetic route in organic chemistry, producing polymers through reactions that eliminate small molecules. In polyester formation, the characteristic link is the ester functional group, created by the reaction between acid-based groups and alcohol-based groups.
Condensation polymerisation: A polymer-forming reaction in which monomers join and eliminate a small molecule, typically water or hydrogen chloride.
This mechanism distinguishes condensation polymers from addition polymers, where no small molecule is lost.
Polyester formation relies on the nucleophilic attack of the alcohol oxygen on the electrophilic carbonyl carbon of a carboxylic acid or acyl chloride. The nature of the acid-containing monomer influences reaction conditions and by-products.
Key Monomers in Polyester Synthesis
Polyesters typically form from two types of bifunctional monomers:
Dicarboxylic acids (HOOC–R–COOH)
Diols (HO–R′–OH)
Alternatively, diacyl chlorides may replace dicarboxylic acids due to their greater reactivity.
Ester bond: The functional link –COO– formed when a carboxylic acid or acyl chloride reacts with an alcohol group.
Between these monomers, many structural variations arise depending on chain length, substituents, and aromatic or aliphatic character.
After introducing ester bonds, it is essential to note that the strength and flexibility of a polyester depend heavily on monomer structure.
Condensation with Dicarboxylic Acids and Diols
This is the classical pathway for polyester synthesis. Heating a dicarboxylic acid with a diol generates long chains through repeated esterification.
A polyester forms when a diol reacts with a dicarboxylic acid, creating an ester link (-COO-) and eliminating water.

This reaction scheme shows a diol and dicarboxylic acid joining repeatedly to form a polyester via ester linkages, releasing water each time a link forms. The boxed R groups represent the remainder of each monomer incorporated into the polymer backbone. Source
Key features of esterification-based polymerisation:
Requires heating under reflux.
Produces water as the eliminated small molecule.
Proceeds stepwise: monomers → oligomers → polymer chains.
Often uses acid catalysts (e.g., strong mineral acids) to enhance esterification.
Process outline:
Step 1: Diol oxygen acts as a nucleophile toward the protonated carboxyl group.
Step 2: Formation of an ester link and release of water.
Step 3: Repetition of the process, extending the polymer chain.
This route is used industrially for many aliphatic and aromatic polyesters.
Condensation with Diacyl Chlorides and Diols
Using diacyl chlorides increases reactivity and removes the need for high-temperature conditions. This method is widely applied in producing high-performance polyesters.
Acyl chloride: A reactive derivative of a carboxylic acid containing the –COCl group, which readily forms esters and amides.
Unlike acid–diol polymerisation, this route eliminates hydrogen chloride (HCl) rather than water. The reaction is fast and often occurs at room temperature.
A normal explanatory sentence should follow the definition, so it is useful to stress that handling HCl requires appropriate safety measures due to its corrosive nature.
If a dioyl chloride is used instead of a dicarboxylic acid, the same ester links form but HCl is eliminated rather than water.
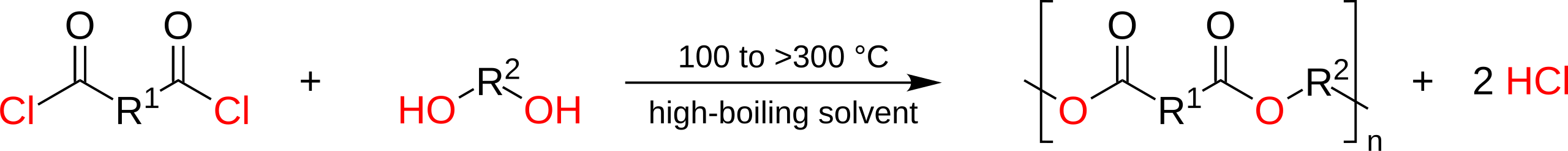
This scheme shows polyester formation from a diacyl chloride and a diol, with hydrogen chloride released as the condensation by-product. The note about ‘no catalyst’ goes beyond OCR requirements, but the HCl elimination is syllabus-relevant. Source
Formation of Polyester Repeat Units
Students must be able to identify and construct repeat units from monomers. The repeat unit contains the ester linkage and retains the residual carbon skeletons of the monomers.
To predict a polyester repeat unit:
Remove the small molecule (H₂O or HCl) formed during polymerisation.
Connect the monomer fragments through –COO– links.
Ensure the repeating pattern continues in both directions.
Important structural features:
If monomers are symmetrical, the repeat unit is straightforward.
Asymmetrical monomers require attention to connectivity.
Aromatic monomers introduce rigidity; aliphatic monomers add flexibility.
A familiar example is poly(ethylene terephthalate) (PET), made from ethane-1,2-diol and benzene-1,4-dicarboxylic acid (terephthalic acid).
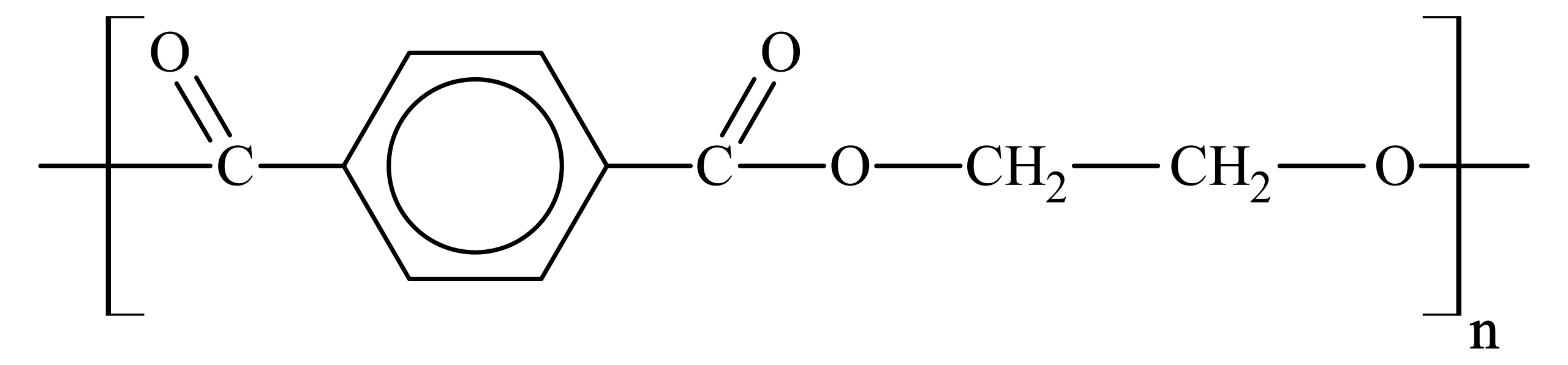
This diagram shows the repeating structure of polyethylene terephthalate (PET), highlighting the ester (-COO-) links between diol- and diacid-derived fragments. It illustrates how monomer units are incorporated into a continuous polymer chain. Source
Influence of Monomer Choice on Polymer Properties
Polyesters vary widely depending on the monomers selected:
Aromatic dicarboxylic acids produce rigid, high-melting polymers suitable for fibres.
Aliphatic acids yield more flexible, biodegradable materials.
Diols with longer chains reduce intermolecular forces, lowering melting point.
Branching groups disrupt packing and reduce crystallinity.
These structural considerations are essential for understanding the practical applications of polyester materials.
Industrial and Laboratory Preparation Conditions
Polyester synthesis conditions differ depending on whether acids or acyl chlorides are used.
When using dicarboxylic acids:
Heating under reflux is required.
Removal of water drives the equilibrium towards polymer formation.
Catalysts are frequently employed.
High temperatures enhance chain length.
When using diacyl chlorides:
Reaction is rapid, often at room temperature.
No catalyst is typically needed.
HCl must be safely vented or neutralised.
Ideal for producing polymers with high molecular masses.
Hydroxycarboxylic Acids as Single-Monomer Sources
Although the OCR specification emphasises acids/acyl chlorides with alcohols or diols, it is also relevant to recognise that monomers containing both –COOH and –OH groups can form polyesters alone.
These molecules undergo self-condensation, producing polymers with repeat units derived from a single monomer. This maintains curriculum alignment without expanding beyond the required reactions.
Key Learning Points for OCR A-Level Chemistry
Polyesters form by condensation polymerisation through ester bond formation.
Dicarboxylic acids or acyl chlorides react with alcohols or diols to form polymer chains.
Water or hydrogen chloride is eliminated each time an ester group forms.
Predicting repeat units requires an understanding of monomer connectivity and eliminated molecules.
Reaction conditions differ significantly between acid–diol and acyl chloride–diol methods.
FAQ
Each monomer must have two functional groups so it can react at both ends and form long polymer chains.
If a monomer had only one functional group, it would terminate the chain, preventing polymer growth beyond a short molecule.
Bifunctional monomers ensure continuous chain extension in both directions during condensation polymerisation.
Condensation polymerisation is an equilibrium process when dicarboxylic acids are used.
Removing the small molecule shifts the equilibrium towards polymer formation.
This is often achieved by:
Heating under reflux
Using excess monomer
Removing water as it forms
These methods increase polymer chain length.
Acyl chlorides contain a highly polar carbon–chlorine bond.
This makes the carbonyl carbon more susceptible to nucleophilic attack by alcohol groups.
As a result:
Reactions occur more rapidly
Lower temperatures can be used
No acid catalyst is usually required
Longer polymer chains lead to stronger intermolecular forces between chains.
This results in:
Higher melting points
Greater tensile strength
Increased durability
Shorter chains produce materials that are softer and more flexible.
Aromatic rings are rigid, flat structures that restrict rotation in the polymer backbone.
This limits flexibility and allows chains to pack closely together.
As a result, aromatic polyesters typically have:
Higher melting points
Increased rigidity
Greater resistance to deformation
Practice Questions
Describe what is meant by condensation polymerisation in the formation of polyesters.
(2 marks)
Award one mark for each correct point:
States that monomers join together to form a polymer. (1)
States that a small molecule such as water or hydrogen chloride is eliminated. (1)
A polyester is made by reacting a diol with a dicarboxylic acid.
(a) Describe how ester links are formed during this reaction.
(b) State the small molecule eliminated during this type of polymerisation.
(c) Explain how the use of a diacyl chloride instead of a dicarboxylic acid changes the reaction conditions or products.
(5 marks)
(a) Ester link formation (3 marks)
Award one mark for each correct point:
Reaction occurs between the –OH group of the diol and the –COOH group of the dicarboxylic acid. (1)
Formation of an ester linkage (–COO–) between monomer units. (1)
Reaction repeats to form a long-chain polymer. (1)
(b) Eliminated molecule (1 mark)
Water is produced as the small molecule eliminated. (1)
(c) Use of a diacyl chloride (1 mark)
Hydrogen chloride is eliminated instead of water or the reaction occurs more readily / at lower temperature. (1)
Maximum total: 5 marks

