OCR Specification focus:
‘From a polymer section, identify required monomers and whether addition or condensation formed it.’
Introduction
Understanding how to deduce monomers from a polymer structure is essential in polymer chemistry, allowing chemists to interpret polymer formation pathways and recognise key functional groups.
Identifying Monomers from Polymer Structures
When analysing a polymer, the primary goal is to determine the monomer(s) originally used to construct the chain. The polymer structure provides structural clues that reveal how the repeat unit was formed and which type of polymerisation occurred. OCR expects you to recognise whether the polymer came from addition polymerisation or condensation polymerisation, as this directly informs how monomers must be reconstructed.
Determining the Polymerisation Type
The first step is to establish whether the polymer was formed by addition polymerisation or condensation polymerisation. This distinction narrows down the possible monomers dramatically.
Addition polymerisation involves alkenes adding together without the loss of small molecules.
Condensation polymerisation involves monomers joining while eliminating small molecules such as H₂O or HCl.
Recognising Addition Polymer Structures
Addition polymers contain a carbon backbone composed solely of single bonds between carbon atoms. This arises from the opening of a C=C double bond in alkene monomers.
Addition Polymerisation: A polymerisation reaction in which unsaturated monomers with C=C double bonds join to form a long chain without losing small molecules.
In practice, the polymer repeat unit resembles the original alkene, but with the double bond removed. Recognising this pattern is key to reconstructing the monomer.
Reconstructing Monomers for Addition Polymers
To identify the monomer from an addition polymer:
Locate the repeat unit—commonly shown in square brackets with an “n” suffix.
Identify the atoms directly bonded along the polymer chain.
Reinstate the C=C bond between the carbons that form the polymer backbone.
Ensure any substituents present in the repeat unit become substituents on the new alkene monomer.
A single monomer usually gives the polymer because addition polymers typically derive from one type of alkene.
For an addition polymer, you can usually recover the monomer by converting the C–C single bond in the repeat unit back into a C=C double bond (keeping the same side-group pattern).
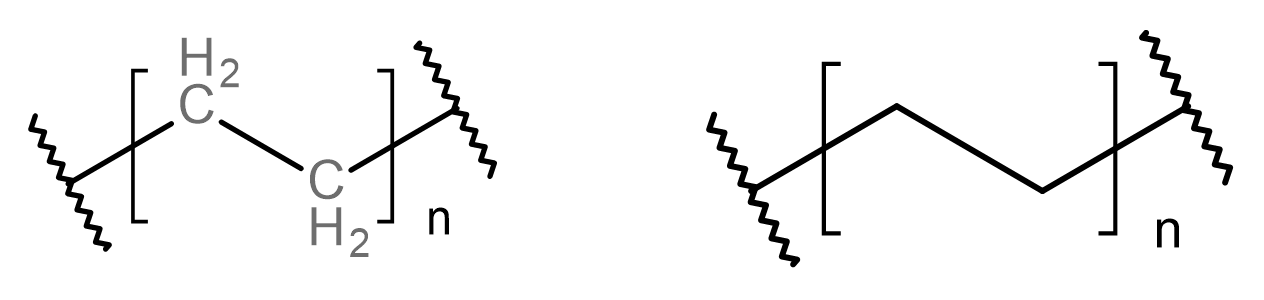
This scheme shows ethene forming poly(ethene) by addition polymerisation, producing a repeat unit written in brackets with n. Reversing this process allows the alkene monomer to be identified by reinstating the C=C bond. Source
Recognising Condensation Polymer Structures
Condensation polymers contain characteristic linkages, typically esters (–COO–) in polyesters or amides (–CONH–) in polyamides. The repeat unit includes atoms that originated from two different monomers, joined with the loss of a small molecule.
Condensation Polymerisation: A polymerisation process in which monomers join together to form a polymer and eliminate small molecules, such as water or hydrogen chloride.
These functional group linkages are the single most important indicator that the polymer was produced by condensation.
A normal sentence must appear here before inserting any further structured elements, ensuring clarity in the differentiation between polymerisation routes.
Reconstructing Monomers for Condensation Polymers
To identify monomers used in condensation polymerisation, locate the bond that formed during polymerisation and determine which atoms were contributed by each monomer.
Typical steps include:
Identify the linkage (e.g., ester or amide).
Break the repeat unit across the linkage.
Add back the atoms removed during polymerisation (commonly H⁺ and OH⁻ or H and Cl).
Deduce whether monomers were:
Diacids and diols (polyesters)
Acyl chlorides and diols (polyesters)
Diacids and diamines (polyamides)
Acyl chlorides and diamines (polyamides)
Breaking Down a Polyester Structure
Polyesters contain the ester linkage, identifiable by –COO–. To find monomers:
Break the polymer at the oxygen–carbonyl bond.
Restore –OH to the carbonyl carbon (forming a carboxylic acid or acyl chloride derivative).
Restore –H to the oxygen atom (forming an alcohol or diol).
These reconstructed molecules correspond to the original monomers used in the condensation process.
For a polyester, split the repeat unit at the ester link (–COO–) to regenerate the two monomers: a diol (–OH ends) and a dicarboxylic acid (–COOH ends).
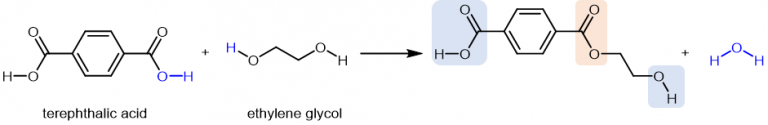
This diagram shows a diacid reacting with a diol to form a polyester via condensation polymerisation, producing ester links and water. The highlighted linkage helps identify where the polymer chain is split to recover the original monomers. Source
Breaking Down a Polyamide Structure
Polyamides contain the amide linkage –CONH–, a key structural indicator. To deduce monomers:
Break the polymer at the C–N bond of the amide.
Restore –OH to the carbonyl carbon.
Restore –H₂N to the nitrogen atom.
Identify the resulting molecules as the dicarboxylic acid (or acyl chloride) and the diamine.
If the polymer contains an amide link (–CONH–), it is a polyamide and the monomers are a diamine and a dicarboxylic acid (or a diacyl chloride).

This scheme illustrates polyamide formation from a diamine and a dicarboxylic acid, with amide links joining the monomers. When working backwards, splitting at each –CONH– linkage reveals the original diamine and diacid structures. Source
Considering Monomer Functionality
Monomers in condensation polymerisation are often difunctional, allowing chain growth at both ends. Common functionalities include:
–COOH (carboxylic acids)
–OH (alcohols)
–NH₂ (amines)
–COCl (acyl chlorides)
Recognising these groups within reconstructed fragments helps confirm the correct monomers.
Identifying Multiple Monomers
Some polymers are formed from two distinct monomers, while others derive from a single bifunctional monomer.
For example:
A polymer containing alternating fragments likely originates from two complementary monomers.
A polymer with a uniform repeating structure may originate from one monomer containing two reactive groups (e.g., an amino acid forming a polyamide).
Using Structural Clues Effectively
When identifying monomers from a polymer structure, focus on:
Functional group patterns (ester or amide linkages)
Backbone composition (all single bonds for addition polymers)
Symmetry of the repeat unit
Presence or absence of heteroatoms within the chain
Direction of polymer growth based on functional group placement
Importance for Organic Synthesis
Understanding how to determine monomers from a polymer is essential for synthetic planning, allowing chemists to identify starting materials and predict product properties. This skill supports wider OCR organic synthesis content by linking functional group transformations to macromolecular structure.
FAQ
Condensation polymerisation relies on monomers reacting at both ends to allow chain growth. If a monomer had only one functional group, polymer chains would stop growing.
Difunctional monomers ensure:
Continuous extension of the polymer chain
Formation of long molecules with repeating units
Proper linking between alternating monomers in the structure
This is why diols, dicarboxylic acids, diamines, and diacyl chlorides are commonly used.
This can often be deduced from the symmetry of the repeat unit.
Symmetrical repeat units often indicate a single bifunctional monomer
Alternating or asymmetrical repeat units usually suggest two different monomers
Careful inspection of functional groups on either side of the linkage helps confirm this.
Acyl chlorides are more reactive than carboxylic acids, allowing polymerisation to occur more readily and at lower temperatures.
Additional features include:
Faster reaction rates
Release of hydrogen chloride instead of water
Easier formation of ester or amide links
This can be useful in industrial polymer synthesis.
No, the direction does not affect the final answer as long as the correct bond is broken and the correct functional groups are restored.
What matters most is:
Identifying the correct linkage
Restoring the appropriate atoms lost during polymerisation
Ensuring chemically sensible monomer structures
Multiple valid drawings may represent the same monomers.
Addition polymers typically come from a single alkene monomer and do not involve the loss of small molecules.
This means:
Only one monomer needs to be identified
The backbone structure directly reflects the original alkene
Reinstating a C=C bond usually reveals the monomer immediately
Condensation polymers require more careful analysis of linkages and functional groups.
Practice Questions
A polymer has the repeating unit shown below:
[–CH2–CH(CH3)–]n
(a) State the type of polymerisation used to form this polymer.
(b) Draw and name the monomer used to make this polymer.
(2 marks)
(a)
Correctly states addition polymerisation.
1 mark
(b)
Correct alkene structure drawn with a C=C double bond.
Correct identification or name of the monomer as propene.
1 mark
A section of a polymer chain is shown below and contains the linkage –COO– within the repeat unit.
(a) State the type of polymerisation used to form this polymer.
(b) Identify the functional group present in the polymer linkage.
(c) Deduce the two different monomers used to make this polymer and state the functional group present in each monomer.
(5 marks)
(a)
Correctly states condensation polymerisation.
1 mark
(b)
Correct identification of the ester functional group.
1 mark
(c)
Correct identification of a diol as one monomer.
1 markCorrect identification of a dicarboxylic acid (or acyl chloride) as the second monomer.
1 markCorrect functional groups stated for both monomers (–OH and –COOH or –COCl).
1 mark
Maximum: 5 marks

