OCR Specification focus:
‘Ester groups in polyesters undergo acid and base hydrolysis to smaller molecules.’
Polyester hydrolysis involves breaking the ester functional group within the polymer backbone using acidic or alkaline conditions, producing smaller, identifiable organic molecules useful in analysis and synthesis.
Hydrolysing Polyesters
Polyesters are condensation polymers formed through reactions between carboxylic acids (or acyl chlorides) and alcohols, creating long chains connected by ester linkages. These ester bonds can be cleaved by hydrolysis, a reaction in which water, often assisted by acid or base, breaks chemical bonds. In this subsubtopic, the focus is on how ester groups within polyesters behave during hydrolysis and the types of products formed under different reaction conditions. Since hydrolysis effectively reverses the original condensation reaction, the products correspond to the monomers or closely related derivatives.
The Ester Functional Group in Polyesters
An ester group is the functional group –COO– found in both small organic molecules and within polyester chains. Hydrolysis targets this linkage, breaking the polymer into smaller fragments.
Polyesters contain repeating ester linkages (–COO–) that can be cleaved by hydrolysis, breaking long chains into smaller molecules.
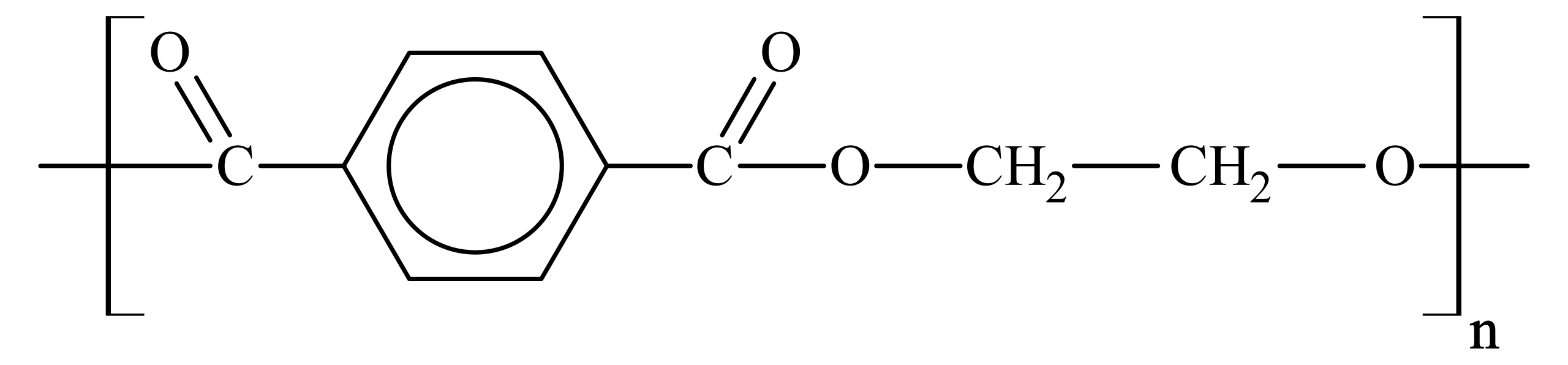
This diagram shows the repeat unit of a polyester (poly(ethylene terephthalate), PET), highlighting the ester linkage (–COO–) within the polymer chain. Hydrolysis targets this bond, breaking the polymer into smaller molecules. Source
Ester Hydrolysis: The chemical process in which an ester bond is broken by water, producing an alcohol and a carboxylic acid or their ionic forms.
In polyesters, the cleavage of multiple ester bonds results in numerous smaller molecules rather than a single reaction product.
Acid Hydrolysis of Polyesters
Acid hydrolysis is the reaction of polyesters with hot aqueous acids, typically dilute hydrochloric or sulphuric acid. This process is generally slower than alkaline hydrolysis but gives fully protonated products. Under acidic conditions, the ester oxygen is protonated, making the carbonyl carbon more susceptible to nucleophilic attack by water.
Mechanistic Overview of Acid Hydrolysis
Although the detailed mechanism is not required by the specification, the essential steps can be summarised through key ideas:
Protonation of the ester group, increasing electrophilicity.
Nucleophilic attack by water.
Breakdown of the tetrahedral intermediate to form the products.
Products of Acid Hydrolysis
Acid hydrolysis of polyesters yields:
Alcohol molecules derived from the diol used to form the polyester.
Carboxylic acids corresponding to the dicarboxylic acid or diacyl chloride monomers.
Under acidic conditions, hydrolysis converts each ester linkage into a carboxylic acid group and an alcohol group.
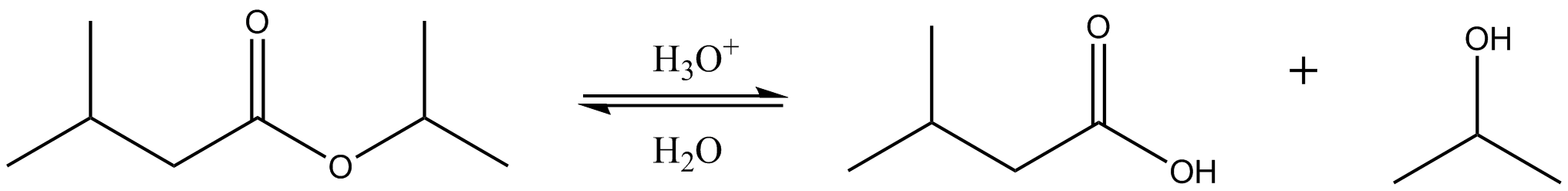
This reaction scheme illustrates acid-catalysed hydrolysis of an ester, forming a carboxylic acid and an alcohol. The same reaction occurs repeatedly along a polyester chain wherever an ester linkage is present. Source
These products match the monomer types used in the original polymer formation, giving insight into polymer composition.
Conditions for Acid Hydrolysis
Students should recognise the typical features:
Hot, aqueous acidic conditions.
Reflux heating to ensure complete breakdown of the polymer.
Reaction proceeds to completion because products are not ionic, so equilibrium favours hydrolysis at high temperature and extended reaction times.
Alkaline Hydrolysis of Polyesters
Alkaline hydrolysis, also referred to as base hydrolysis or saponification, is the reaction of polyesters with hot aqueous alkali such as sodium hydroxide. This process is generally faster than acid hydrolysis and produces ionic products.
Saponification: Base-catalysed hydrolysis of an ester forming a carboxylate ion and an alcohol.
A normal sentence must appear here to maintain flow before introducing other formatted material, as required.
Mechanistic Overview of Alkaline Hydrolysis
The specification does not require students to memorise the full mechanism, but the key conceptual steps include:
Hydroxide ions attacking the carbonyl carbon directly because the ester is sufficiently electrophilic.
Formation of a tetrahedral intermediate.
Elimination of an alkoxide ion, followed by proton transfer to form the final alcohol.
Products of Alkaline Hydrolysis
Base hydrolysis produces:
Alcohol molecules corresponding to the diol monomer.
Carboxylate salts, rather than carboxylic acids, because the carboxylic acid formed initially is deprotonated by the excess hydroxide.
Under alkaline hydrolysis (hot aqueous NaOH or KOH), each ester linkage forms an alcohol and a carboxylate salt.
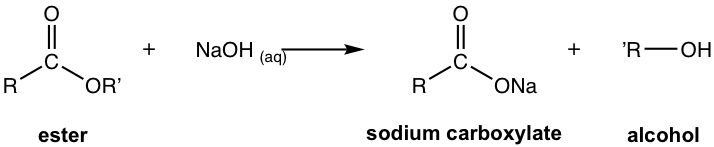
This diagram shows base-promoted ester hydrolysis producing an alcohol and a carboxylate ion. The soap-making context of saponification is beyond OCR requirements, but the reaction outcome matches alkaline polyester hydrolysis. Source
These salts remain water-soluble, making purification and analysis simpler in laboratory contexts.
Conditions for Alkaline Hydrolysis
Important features include:
Hot aqueous alkali, typically NaOH or KOH.
Faster reaction due to increased nucleophilicity of hydroxide ions.
Irreversible reaction because the carboxylate salt product is stabilised and does not reform the ester.
Comparing Acid and Base Hydrolysis of Polyesters
To help consolidate understanding of polyester behaviour under different conditions, it is essential to compare the outcomes of each method. Hydrolysis always breaks the ester groups, but the identity of the carboxyl-containing product varies.
Key Distinctions
Acid hydrolysis produces carboxylic acids and alcohols.
Base hydrolysis produces carboxylate salts and alcohols.
Acid hydrolysis is reversible; base hydrolysis is effectively irreversible.
Influence on Polymer Identification
Because the products correspond to the original monomers (or closely related ionic forms), hydrolysis is a useful analytical approach. By identifying the alcohols and acids (or their salts) produced, chemists can deduce:
The nature of the original monomers.
The type of polyester formed.
Whether the polymer was made using diacyl chlorides or dicarboxylic acids.
Importance in Wider Organic Chemistry and Synthesis
Hydrolysis reactions connect the study of polymers with earlier topics involving esters, carboxylic acids, and alcohols. Understanding polyester hydrolysis also supports later synthesis content by illustrating how functional groups behave in large molecules. Furthermore, recognising how hydrolysis conditions influence product type prepares students for broader organic problem-solving involving multi-step transformations.
Practical Considerations
While not a procedural topic, some operational features are valuable for comprehension:
Polyesters must be heated with the appropriate reagent to ensure full hydrolysis.
Reaction time and temperature directly affect the extent of breakdown.
Solid polyesters often require stirring or mechanical agitation to maximise contact with aqueous reagents.
These ideas help contextualise the chemical principles behind hydrolysing polyesters.
FAQ
Polyesters are large, solid macromolecules with many ester linkages packed closely together. This reduces the accessibility of individual ester groups to water, acid, or hydroxide ions.
In addition, the polymer chains are often crystalline or semi-crystalline, which further slows reaction rates. Prolonged heating under reflux increases molecular motion and ensures sufficient contact between the polymer and the hydrolysing reagent.
The rate of hydrolysis depends on how easily reagents can reach the ester linkages.
Factors that slow hydrolysis include:
Rigid aromatic rings near the ester group
High crystallinity of the polymer
Strong intermolecular forces between chains
More flexible polyesters with aliphatic segments tend to hydrolyse more readily than rigid aromatic polyesters.
In alkaline hydrolysis, hydroxide ions react with the ester linkage and the resulting carboxylic acid is immediately deprotonated to a carboxylate ion.
This removes the acid product from equilibrium. Because the carboxylate ion is stabilised and does not readily reform the ester, the reaction proceeds to completion without relying on excess water.
Yes, hydrolysis can be used as a chemical recycling method. By breaking ester linkages, polyesters can be converted back into smaller molecules related to their original monomers.
These products can then be:
Purified
Reused to synthesise new polymers
Used as starting materials in other chemical processes
This approach reduces waste and supports more sustainable polymer use.
Carboxylate salts are ionic compounds. The negatively charged carboxylate ion interacts strongly with water molecules through ion–dipole attractions.
This high solubility prevents precipitation and keeps the products dispersed in solution. As a result, the polymer continues to break down without forming a solid barrier that would slow further hydrolysis.
Practice Questions
A polyester contains repeating ester linkages in its polymer chain. Describe what happens to these ester linkages when the polyester is hydrolysed under acidic conditions.
(2 marks)
1 mark: States that the ester linkage is broken / cleaved by hydrolysis.
1 mark: Identifies that the products formed are a carboxylic acid and an alcohol (both must be mentioned).
A polyester is heated under reflux with aqueous sodium hydroxide.
(a) Name the type of hydrolysis that occurs.
(b) Describe the products formed from the ester linkages in the polyester.
(c) Explain why this reaction is considered irreversible.
(5 marks)
(a) (1 mark)
1 mark: Identifies the reaction as alkaline hydrolysis / base hydrolysis / saponification.
(b) (2 marks)
1 mark: States that alcohol molecules are formed.
1 mark: States that carboxylate salts (or carboxylate ions) are formed.
(c) (2 marks)
1 mark: Explains that the carboxylic acid formed is deprotonated to a carboxylate ion by the alkali.
1 mark: Explains that the carboxylate salt does not readily reform the ester, making the reaction effectively irreversible.

