OCR Specification focus:
‘React haloalkanes with CN– in ethanol; outline nucleophilic substitution mechanism to form nitriles.’
Haloalkanes react with cyanide ions via nucleophilic substitution, enabling valuable carbon–carbon bond formation. This reaction lengthens carbon chains and provides key synthetic intermediates.
The Role of Cyanide in Carbon–Chain Extension
The reaction between haloalkanes and cyanide ions (CN⁻) is a core method for increasing carbon chain length in organic synthesis. It provides access to nitriles, versatile intermediates in later transformations such as reduction to amines or hydrolysis to carboxylic acids. The OCR specification emphasises understanding the mechanism, the conditions, and why CN⁻ behaves as an effective nucleophile.
Nature of the Cyanide Ion
The cyanide ion acts as a nucleophile, meaning it donates a lone pair to an electron-deficient carbon.
Nucleophile: A species that donates an electron pair to an electron-deficient atom to form a new covalent bond.
Because CN⁻ carries a negative charge and has a highly electron-rich carbon atom, it readily attacks the δ⁺ carbon in a polar C–X bond (X = halogen). This allows strong C–C bond formation, one of the major goals of synthetic organic chemistry.
A single sentence must occur here to separate definition blocks. The cyanide ion's reactivity is also important for determining suitable reaction conditions.
Required Conditions for Reaction
For the substitution to occur efficiently, students must remember the specific conditions highlighted in the OCR specification.
Solvent and Reagent Choices
Potassium cyanide (KCN) or sodium cyanide (NaCN) provides the CN⁻ ion.
The reaction mixture is heated under reflux in ethanol, not water.
Reflux: A technique in which reaction vapours condense and return to the reaction mixture, allowing heating without loss of volatile substances.
A normal sentence separating blocks helps maintain clarity. Ethanol is used because water would encourage elimination or hydrolysis instead of nucleophilic substitution.
Safety Considerations
Though not a specification requirement, understanding cyanide hazards reinforces why the reaction must be handled carefully: CN⁻ is highly toxic and requires appropriate laboratory precautions.
The Nucleophilic Substitution Mechanism
The reaction between haloalkanes and CN⁻ follows the nucleophilic substitution pathway. For most haloalkanes at A-Level, this is examined through the SN2 mechanism, although mechanisms are not always assessed in mechanistic depth.
Polar Nature of the C–X Bond
The carbon–halogen bond is polar, with carbon carrying a δ⁺ charge and the halogen carrying a δ⁻ charge. This polarisation attracts the nucleophile, which initiates the reaction.
Electrophile: A species that accepts an electron pair during bond formation.
This sentence separates the content effectively before continuing. In SN2 reactions, the nucleophile attacks from the opposite side of the leaving group.
Mechanistic Steps in SN2
CN⁻ approaches the carbon atom opposite the halogen (backside attack).
CN⁻ forms a new C–C bond, while the C–X bond breaks simultaneously.
The leaving group (X⁻) departs, producing a nitrile (R–C≡N).
Leaving group: An atom or group that departs with an electron pair during substitution.
A normal sentence follows here. This concerted one-step mechanism results in inversion of configuration at a chiral centre, though stereochemistry is not a major focus in this subsubtopic.
Reaction Outcome and Product Formation
The main product of this reaction is a nitrile, and its formation marks the creation of a new carbon–carbon bond.
Formation of Nitriles
A nitrile contains the –C≡N functional group, which can undergo further reactions encountered elsewhere in the specification (e.g., reduction and hydrolysis). For this subsubtopic, the key point is that nucleophilic substitution transforms the haloalkane into a nitrile by replacing the halogen atom with CN⁻.
Haloalkanes react with cyanide ions, CN⁻, in ethanol to form nitriles (R–C≡N) via nucleophilic substitution.
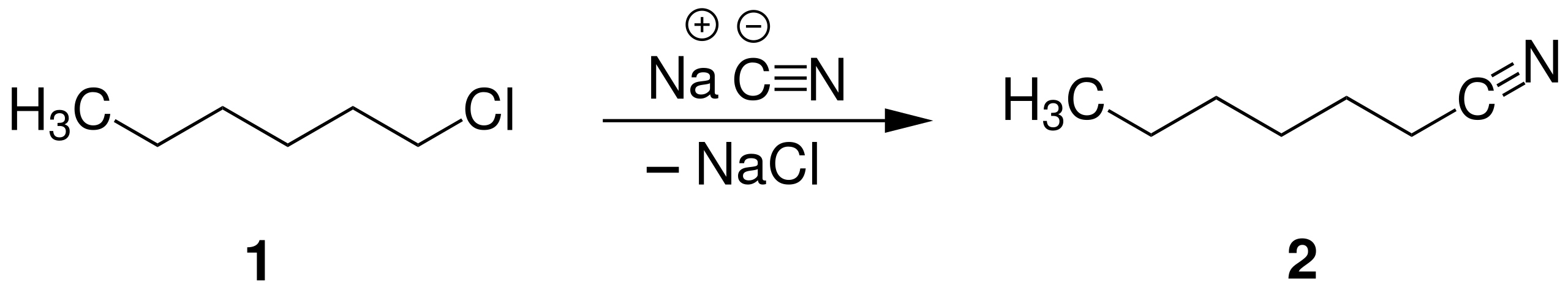
This reaction scheme shows a haloalkane undergoing nucleophilic substitution with cyanide to form a nitrile, increasing the carbon chain length by one carbon. The halogen leaves as a halide ion while the C≡N group is introduced. The solvent ethanol is not explicitly shown. Source
Nucleophilic Substitution (Haloalkane + CN⁻) = R–C≡N + X⁻
R = Alkyl group
X = Halide ion
Here is a sentence separating the equation from other blocks. The reaction greatly increases synthetic flexibility because nitriles act as branching points for multiple pathways.
Conditions Favouring Substitution
To maximise substitution rather than elimination:
Use ethanolic rather than aqueous solvent.
Maintain reflux conditions to ensure reaction completion.
Use excess CN⁻ to push the equilibrium and discourage competing reactions.
These considerations ensure that the major product remains the nitrile rather than undesirable by-products.
Importance in Organic Synthesis
Haloalkane–CN⁻ substitution is repeatedly used in A-Level synthetic pathways because it introduces new carbon atoms in a single step. The product nitrile:
lengthens the carbon chain
provides routes to amines, acids, and other valuable compounds
fits easily into multi-stage synthesis questions set by OCR
In an SN2 mechanism, CN⁻ attacks the δ⁺ carbon from the side opposite the C–X bond while the halide leaves in the same step.
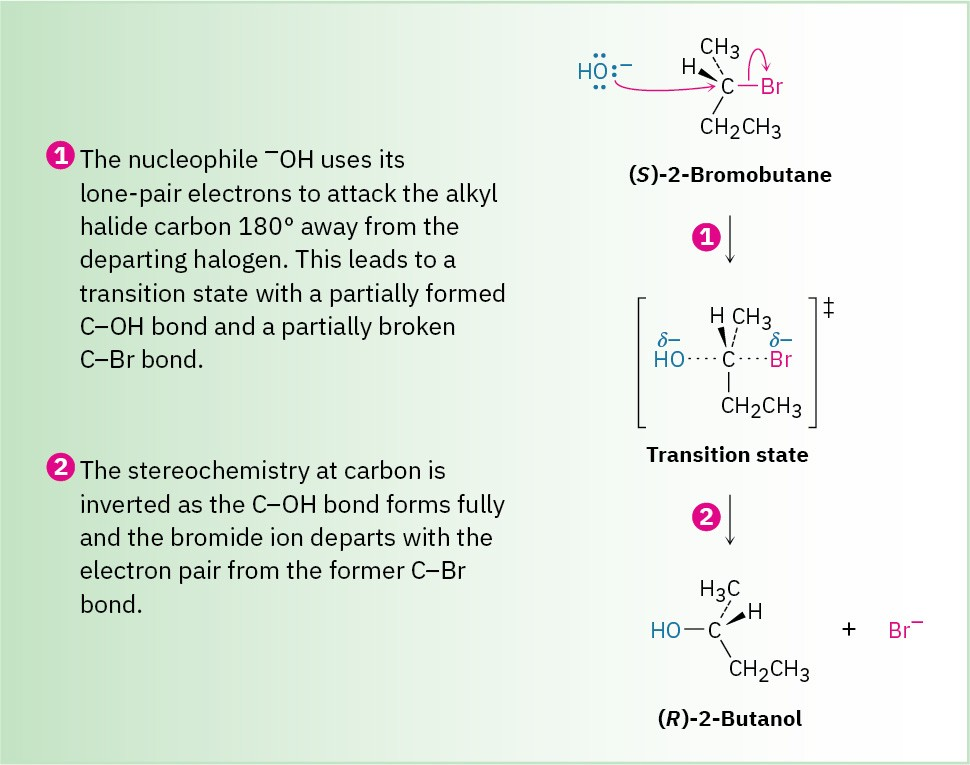
This diagram illustrates the SN2 substitution mechanism, showing a nucleophile attacking from the opposite side of the leaving group in a single step. Bond formation and bond breaking occur simultaneously through a transition state. The nucleophile shown is OH⁻ rather than CN⁻, but the mechanistic pattern is identical. Source
The specification requires students to understand both the reagents and the outline mechanism, as well as the conditions required to generate nitriles reliably.
FAQ
Reaction rate depends on steric hindrance around the carbon bonded to the halogen.
Primary haloalkanes react fastest due to minimal steric hindrance
Secondary haloalkanes react more slowly
Tertiary haloalkanes react very slowly or not at all via SN2
This explains why the reaction is typically limited to primary and some secondary haloalkanes.
The reaction between haloalkanes and CN⁻ is relatively slow at room temperature.
Heating increases the kinetic energy of particles, leading to more frequent successful collisions. Reflux allows the reaction to be heated for extended periods without loss of volatile organic compounds, ensuring sufficient conversion to the nitrile product.
Cyanide ions are strong nucleophiles but relatively weak bases.
Elimination reactions require strong bases, such as hydroxide ions, especially in hot ethanolic conditions. Using CN⁻ favours substitution because it preferentially attacks the electrophilic carbon rather than removing a proton from a neighbouring carbon.
Nitriles are valuable because the –C≡N group can be transformed into multiple functional groups.
Reduction produces primary amines
Acidic hydrolysis produces carboxylic acids
This versatility allows chemists to design multi-step synthetic routes efficiently while controlling carbon chain length.
Although CN⁻ contains both carbon and nitrogen atoms, the nucleophilic site is the carbon atom, not the nitrogen.
The carbon end carries greater electron density and is less electronegative than nitrogen, making it more reactive towards δ⁺ carbon atoms in haloalkanes. This leads to formation of a C–C bond, which is essential for carbon-chain extension in synthesis.
Practice Questions
A bromoalkane reacts with potassium cyanide in ethanol under reflux.
State the type of reaction taking place and name the organic product formed.
(2 marks)
Identifies the reaction as nucleophilic substitution (1 mark)
States that a nitrile is formed (or correctly names a nitrile product) (1 mark)
A primary haloalkane reacts with cyanide ions in ethanol.
(a) Describe the nucleophilic substitution mechanism that occurs in this reaction.
(b) Explain why ethanol is used as the solvent rather than water.
(5 marks)
(a) Mechanism description (3 marks)
Cyanide ion acts as a nucleophile and attacks the carbon atom bonded to the halogen (1 mark)
Carbon–halogen bond breaks as the halide ion leaves (1 mark)
Bond formation and bond breaking occur in a single step (or describes SN2 mechanism) (1 mark)
(b) Solvent choice (2 marks)
Ethanol does not supply hydroxide ions / is not aqueous (1 mark)
Water would favour hydrolysis or elimination rather than substitution with CN⁻ (1 mark)

