OCR Specification focus:
‘Add HCN to carbonyls via CN– attack then protonation to make hydroxynitriles.’
Introduction
Hydrogen cyanide adds across aldehyde and ketone C=O bonds through nucleophilic addition, where cyanide ions attack the electrophilic carbonyl carbon, forming useful hydroxynitrile intermediates.
Nucleophilic Addition of HCN to Carbonyl Compounds
Hydrogen cyanide reacts with aldehydes and ketones via nucleophilic addition, a key carbon–carbon bond-forming reaction used widely in synthetic chemistry. The mechanism occurs through attack by the cyanide ion (CN⁻) followed by protonation to generate hydroxynitriles, which contain both a hydroxyl (–OH) and nitrile (–C≡N) group.
Carbonyl Reactivity: Why the C=O Bond Is Susceptible
The carbonyl group is polar, with the carbon atom carrying a partial positive charge and the oxygen atom a partial negative charge. This polarity makes aldehydes and ketones highly susceptible to nucleophilic attack.
Nucleophile: A species that donates a lone pair of electrons to form a covalent bond.
Because the cyanide ion possesses a lone pair on carbon and carries a negative charge, it acts as a strong nucleophile, enabling it to attack the electrophilic carbonyl carbon.
A sentence separating blocks to follow specification rules.
Hydroxynitrile: An organic molecule containing both a nitrile group (–C≡N) and a hydroxyl group (–OH) bonded to the same carbon atom.
Role of Hydrogen Cyanide (HCN) and Its Formation in Situ
Hydrogen cyanide is highly toxic and volatile; therefore, in laboratory settings it is generated in situ by reacting sodium cyanide (NaCN) with dilute acid. This releases the cyanide ion, ensuring safer handling and sufficient nucleophile concentration for the reaction.
Overall Reaction Overview
The reaction converts the carbonyl compound into a hydroxynitrile through two essential mechanistic stages:
Stage 1: Nucleophilic attack by CN⁻
Stage 2: Protonation of the intermediate alkoxide ion
This makes the process highly valuable for increasing molecular complexity and extending carbon chains.
Mechanism of the Reaction
The reaction mechanism between a carbonyl compound and HCN always involves nucleophilic addition, in line with the OCR specification.
Step 1: Nucleophilic Attack by CN⁻
The cyanide ion approaches the carbonyl carbon from the side opposite the oxygen. This attack breaks the C=O π-bond and generates a tetrahedral intermediate.
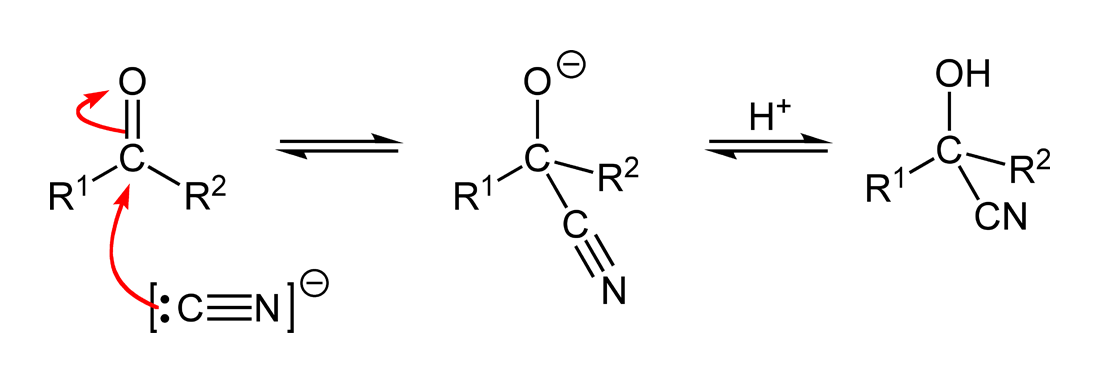
This diagram shows nucleophilic addition of CN⁻ to a carbonyl to form a tetrahedral alkoxide, followed by protonation to give the cyanohydrin (hydroxynitrile). Curly arrows indicate electron-pair movement in each step. Source
Key mechanistic details include:
The lone pair on the cyanide carbon forms a new σ bond with the carbonyl carbon.
The oxygen atom becomes negatively charged, forming an alkoxide ion intermediate.
Attack can occur from either side of the trigonal planar carbonyl group, which allows formation of racemic mixtures if the resulting carbon is chiral.
Step 2: Protonation of the Intermediate
The negatively charged oxygen atom is protonated by HCN or another acidic species in the reaction mixture.
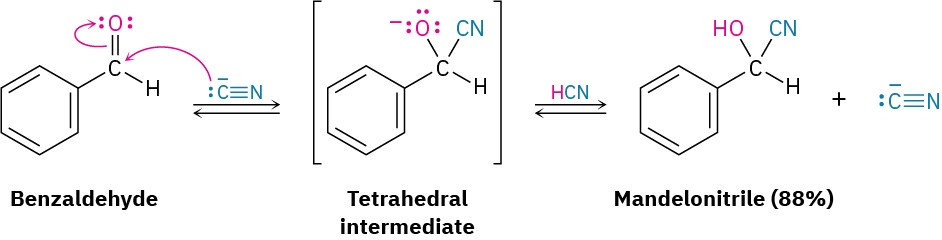
This reaction scheme illustrates CN⁻ addition to benzaldehyde to give a tetrahedral alkoxide intermediate, followed by protonation by HCN to form mandelonitrile. The percentage yield shown is extra detail and not required by the syllabus. Source
This step produces the final hydroxynitrile, which features:
A newly formed C–C bond
An –OH group formed via protonation
A –C≡N group carried over from cyanide
The two-step nature of the mechanism is consistent across all aldehydes and ketones, though aldehydes generally react more readily because of reduced steric hindrance.
Conditions Required for the Reaction
To achieve successful nucleophilic addition of HCN to carbonyl compounds, specific conditions are used:
A source of CN⁻, typically NaCN or KCN
Dilute acid, to generate HCN and to protonate intermediates
Aqueous or ethanolic solvent, depending on solubility needs
Temperature control, usually room temperature to prevent side reactions
These conditions ensure that cyanide ions remain available as nucleophiles throughout the reaction.
Properties and Significance of Hydroxynitriles
Hydroxynitriles are valuable intermediates because they contain two functional groups that can undergo further transformations.
Synthetic Utility
The nitrile group enables:
Hydrolysis to carboxylic acids
Reduction to primary amines
Grignard reactions after suitable transformations
The hydroxyl group additionally supports many common organic reactions such as esterification and oxidation (though the latter must be controlled carefully to avoid degrading the nitrile group).
Stereochemical Outcomes
When the carbonyl compound forms a chiral centre during CN⁻ addition, the product will generally exist as a racemic mixture. This is due to:
The planar carbonyl allowing attack from either the top or bottom
Each pathway being equally likely under non-chiral conditions
Because the OCR specification emphasises understanding these stereochemical consequences, students should associate HCN addition with racemate formation whenever new chiral centres emerge.
Reaction Pathway Overview in Bullet Format
Carbonyl group is polar and electrophilic at the carbon atom.
CN⁻ acts as a nucleophile, attacking the carbonyl carbon.
The C=O π-bond breaks, forming a tetrahedral intermediate.
The oxygen becomes negatively charged as an alkoxide ion.
Protonation by HCN or acid produces a hydroxyl group.
Final product is a hydroxynitrile, containing both –OH and –C≡N.
If a chiral centre forms, a racemic mixture is obtained.
Applications in Organic Synthesis
The addition of HCN to carbonyls provides a straightforward route to molecules with increased carbon number. Because the nitrile group can be transformed into many other functional groups, this reaction is a foundation for designing multi-step synthetic routes in advanced organic chemistry.
Hydroxynitriles generated by this method appear frequently in synthetic pathways for pharmaceuticals, fragrances, and fine chemicals, making mastery of this mechanism essential for A-Level study and beyond.
FAQ
Hydrogen cyanide is extremely toxic and volatile, making it unsafe to handle directly in a laboratory or exam context.
Instead, it is generated in situ using sodium or potassium cyanide and a dilute acid.
This approach:
Reduces the risk of inhalation
Maintains a steady, low concentration of HCN
Ensures sufficient CN⁻ ions are available for nucleophilic attack
This controlled generation is essential for both safety and reaction efficiency.
Although CN⁻ contains both carbon and nitrogen, the carbon atom is the nucleophilic site.
This is because:
The negative charge is stabilised on carbon
Carbon is less electronegative than nitrogen
Attack via carbon forms a stable C–C bond
Nitrogen attack would not lead to the hydroxynitrile product required by the mechanism.
Aldehydes are generally more reactive than ketones in nucleophilic addition reactions.
Key reasons include:
Less steric hindrance around the carbonyl carbon
Fewer electron-donating alkyl groups, making the carbonyl carbon more electrophilic
This increased accessibility allows CN⁻ to attack more easily in aldehydes.
In the HCN–carbonyl reaction, no atom or group is replaced.
Instead:
CN⁻ adds across the C=O double bond
The π bond breaks, and new σ bonds form
Both CN and H remain in the final product
This satisfies the definition of an addition reaction, not substitution.
When CN⁻ adds to an unsymmetrical carbonyl compound, a new chiral centre may form.
Because:
The carbonyl carbon is planar
CN⁻ can attack from either side
Both mirror-image products form in equal amounts, producing a racemic mixture if no chiral conditions are present.
Practice Questions
Hydrogen cyanide reacts with aldehydes and ketones to form hydroxynitriles.
State the type of mechanism involved in this reaction and name the nucleophile responsible for the initial attack.
(2 marks)
Identifies the mechanism as nucleophilic addition (1 mark)
Correctly names the cyanide ion (CN⁻) as the nucleophile (1 mark)
Describe the mechanism for the reaction of hydrogen cyanide with a carbonyl compound to form a hydroxynitrile.
Your answer should include the role of the cyanide ion and the steps involved in the mechanism.
(5 marks)
States that the reaction occurs by nucleophilic addition to the carbonyl group (1 mark)
Describes attack by CN⁻ on the electrophilic carbonyl carbon (1 mark)
Explains that the C=O π bond breaks and electrons move onto oxygen (1 mark)
Identifies formation of a tetrahedral alkoxide intermediate (1 mark)
Describes protonation of the alkoxide ion to form the hydroxynitrile product (1 mark)

