OCR Specification focus:
‘Hydrolyse nitriles under acidic conditions to form carboxylic acids.’
Acid hydrolysis of nitriles converts the –C≡N functional group into a carboxylic acid, providing a key route for carbon-chain extension and functional group transformation in synthetic chemistry.
Acid Hydrolysis of Nitriles
Acid hydrolysis of nitriles is an essential transformation in organic synthesis, allowing the conversion of the nitrile functional group into a carboxylic acid. According to the OCR specification, nitriles are hydrolysed under acidic conditions to form carboxylic acids, making this reaction a crucial step in many multi-stage synthetic pathways. The reaction is particularly valuable because nitriles themselves are formed through chain-extending reactions, meaning hydrolysis enables the incorporation of an additional carbon into the resulting acid.
Introducing the Nitrile Group
A nitrile contains a carbon–nitrogen triple bond, written as –C≡N, which is strongly polar and reactive towards hydrolysis. The carbon atom is electrophilic, allowing water or hydroxonium ions to attack during hydrolysis.
Nitrile: An organic compound containing the –C≡N functional group, with a polar carbon–nitrogen triple bond susceptible to hydrolysis.
Hydrolysis under acidic conditions provides a controlled and predictable route to form a carboxylic acid, ensuring full conversion of intermediate species.
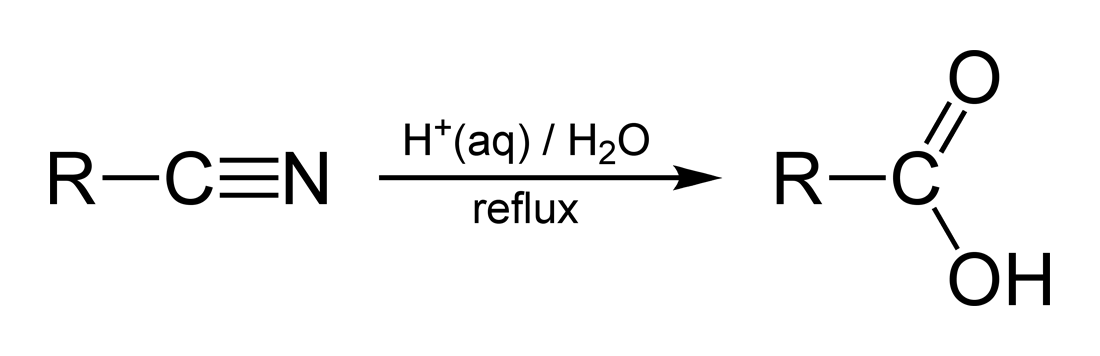
This scheme summarises the conversion of a nitrile (R–C≡N) into a carboxylic acid (R–COOH) using aqueous acid and heat under reflux, highlighting the key conditions required by the OCR specification. Source
Mechanism Overview of Acid Hydrolysis
The hydrolysis proceeds in several steps, ultimately replacing the nitrile’s nitrogen with an oxygen-containing functional group. Although students do not need to memorise every mechanistic arrow, understanding the key transformations improves conceptual clarity.
Stepwise Process of Acid Hydrolysis
Under acidic conditions, nitriles convert into carboxylic acids through the following progression:
Protonation of the nitrile nitrogen increases electrophilicity of the carbon.
Nucleophilic attack by water, forming an imidic acid intermediate.
Tautomerisation to form an amide.
Further acid-catalysed hydrolysis of the amide to produce a carboxylic acid and an ammonium ion.
Hydrolysis: A reaction in which a chemical bond is broken by the addition of water, frequently catalysed by an acid or base.
The formation of an amide intermediate is important conceptually, as it demonstrates that nitrile hydrolysis proceeds through two linked hydrolyses: nitrile → amide → carboxylic acid.
Conditions Required for Acid Hydrolysis
Acid hydrolysis requires prolonged heating and strongly acidic media to ensure complete conversion, as the nitrile group is relatively resistant to attack compared with other functional groups.
Essential Conditions
Concentrated dilute strong acid, typically hydrochloric acid or sulfuric acid
Reflux to maintain consistent reaction temperature
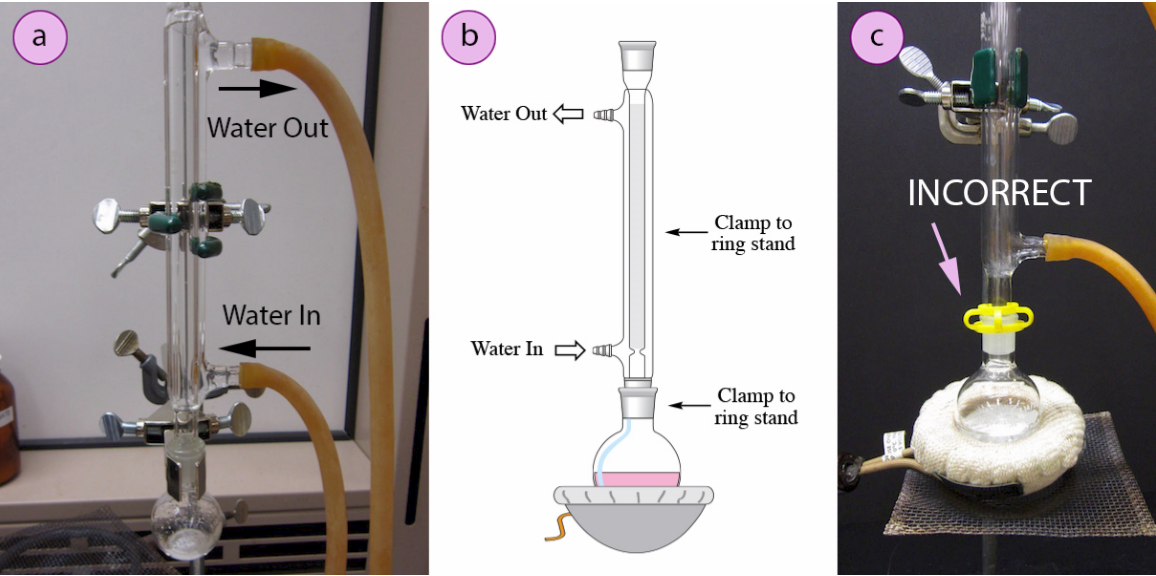
This figure illustrates a standard reflux setup, emphasising correct condenser water flow (in at the bottom, out at the top) to maintain efficient cooling during prolonged heating; the incorrect clamping example provides additional practical context beyond the syllabus. Source
Excess water to drive hydrolysis to completion
Sufficient time for conversion of both the nitrile and the intermediate amide
Products of Acid Hydrolysis
The final organic product is the carboxylic acid, while the nitrogen atom is released as ammonium ions (NH4⁺) when hydrochloric acid is used.
Acid Hydrolysis of a Nitrile (General) = R–C≡N + 2H₂O + H⁺ → R–COOH + NH₄⁺
R = Alkyl chain attached to nitrile group
This equation illustrates the stoichiometric requirement for water and acidic protons, but real reactions often use excess acid to ensure complete hydrolysis.
Why Acid Hydrolysis Is Important in Organic Synthesis
Nitriles are widely used to lengthen carbon chains, and their hydrolysis is a direct route to generating new carboxylic acids with an additional carbon atom. This transformation is often used in synthetic design, especially when routes require strategic functional group interconversions.
Synthetic Utility
Enables formation of carboxylic acids from haloalkanes via nitrile intermediates
Provides access to acids without using strong oxidising agents
Creates versatile intermediates that undergo further reactions such as esterification or reduction
Supports multi-step synthesis planning by linking chain extension to subsequent functional group transformations
Chain extension followed by hydrolysis is an essential strategy in assembling more complex molecules, particularly in pathways outlined in organic synthesis sections of the specification.
Acid Hydrolysis vs Base Hydrolysis (Brief Context)
Although this subsubtopic focuses on acid hydrolysis, understanding its distinction from base hydrolysis strengthens comprehension. Acid hydrolysis yields a carboxylic acid directly, whereas base hydrolysis forms a carboxylate salt, requiring further acidification to produce the free acid. This difference highlights the practical and mechanistic importance of acidic conditions.
A normal sentence must appear here before introducing a definition to satisfy formatting rules.
Carboxylic Acid: An organic compound containing the –COOH functional group, formed by complete hydrolysis of a nitrile under acidic conditions.
Key Features to Remember
Acid hydrolysis converts a nitrile (–C≡N) into a carboxylic acid (–COOH).
Reaction requires acid, heat under reflux, and excess water.
Process proceeds through an amide intermediate before full hydrolysis.
Nitrogen is released as ammonium ions in strongly acidic solutions.
This reaction is central to carbon-chain extension and synthetic design across the OCR A-Level Chemistry specification.
FAQ
The carbon–nitrogen triple bond in a nitrile is very strong and relatively unreactive.
As a result, the nitrile carbon is less easily attacked by water molecules. Strongly acidic conditions and sustained heating under reflux are needed to break this bond and allow complete hydrolysis.
In contrast, ester hydrolysis involves a weaker carbon–oxygen bond, so it occurs more readily under milder conditions.
Hydrolysis of a nitrile occurs in two stages rather than a single step.
First, the nitrile is hydrolysed to an amide. Only then is the amide further hydrolysed to a carboxylic acid.
This happens because the addition of water to the nitrile does not immediately produce a carboxylic acid; structural rearrangement and further hydrolysis are required before the –COOH group forms.
The reaction takes place in strongly acidic solution.
Any ammonia formed during hydrolysis is immediately protonated by excess acid to form ammonium ions, NH4⁺.
This is why ammonium ions are observed as the inorganic nitrogen-containing product instead of free ammonia gas.
Acidic conditions drive the reaction fully towards carboxylic acid formation.
The carboxylic acid produced is stable in acidic solution and does not react further. Excess acid also ensures that intermediate amides are completely hydrolysed, preventing incomplete conversion.
This makes acid hydrolysis a reliable method when a carboxylic acid is the desired final product.
Acid hydrolysis produces the carboxylic acid directly, without forming salts.
This avoids the additional step of acidifying a carboxylate salt, which is required after base hydrolysis.
As a result, acid hydrolysis simplifies purification and is often preferred when designing multi-stage synthetic routes involving nitriles.
Practice Questions
A student hydrolyses a nitrile to form a carboxylic acid.
State the reagents and conditions required for the acid hydrolysis of a nitrile.
(2 marks)
Aqueous acid, e.g. hydrochloric acid or sulfuric acid (1 mark)
Heat under reflux (1 mark)
A nitrile with the formula CH3CH2CN is converted into a carboxylic acid.
(a) Name the organic product formed after acid hydrolysis.
(b) Describe the conditions required for this reaction.
(c) State the inorganic product formed from the nitrogen atom during the reaction.
(5 marks)
(a) Name of organic product (1 mark)
Propanoic acid
(b) Conditions (2 marks)
Aqueous strong acid, e.g. hydrochloric acid or sulfuric acid (1 mark)
Heat under reflux (1 mark)
(c) Inorganic product (2 marks)
Ammonium ions / NH4⁺ (1 mark)
Correct indication that nitrogen forms ammonium under acidic conditions (1 mark)

