OCR Specification focus:
‘Reduce nitriles, e.g. H₂/Ni, to form amines as synthetic intermediates.’
Nitrile reduction is a key synthetic transformation that converts carbon–nitrogen triple bonds into amines, allowing chemists to build longer carbon chains efficiently.
Overview of Nitrile Reduction
Nitriles contain the –C≡N functional group, which can be transformed into amines through reduction. This reaction is an important step in organic synthesis because it enables the conversion of relatively unreactive nitriles into versatile amines that can undergo many further reactions.
In the OCR A-Level Chemistry specification, the emphasis is on understanding:
That nitriles can be reduced to amines
The reagents and conditions used
How this reaction fits into multi-step synthetic routes
The reaction increases the carbon chain length by one carbon compared to the original haloalkane used to make the nitrile, making it valuable in synthesis planning.
Nature of the Functional Group
A nitrile contains a carbon atom triple-bonded to nitrogen. This bond is strong and requires vigorous reducing conditions to break.
Nitrile: An organic compound containing the –C≡N functional group, where carbon is triple bonded to nitrogen.
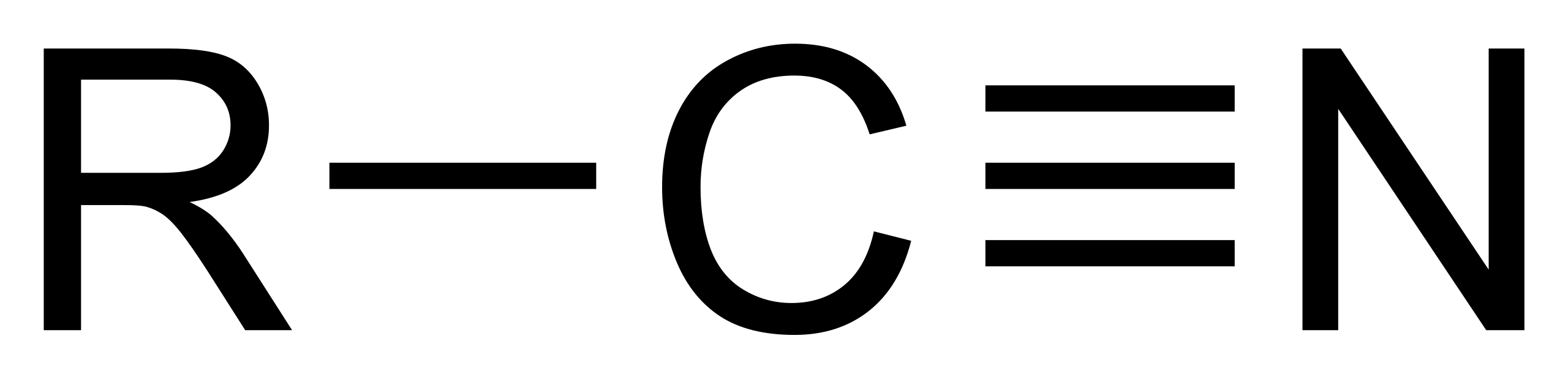
This structure highlights the nitrile functional group, showing the linear C≡N triple bond attached to an organic group (R). Recognising this –C≡N motif is essential before tracking its conversion to –CH₂NH₂ during reduction. Source
During reduction, the triple bond is converted into a single bond, forming a primary amine.
Reduction Products
When a nitrile is reduced, the product is always a primary amine, regardless of the original structure of the nitrile.
Key structural change:
–C≡N becomes –CH₂NH₂
The carbon of the nitrile becomes part of the amine’s alkyl chain
For example:
Ethanenenitrile forms ethylamine
Propanenitrile forms propylamine
This transformation is predictable and consistent, which is why it is frequently used in synthetic pathways.
Reagents and Conditions
The OCR specification requires knowledge of one main reducing system.
Catalytic Hydrogenation
Nitriles are reduced using hydrogen gas in the presence of a nickel catalyst.
Key conditions:
Hydrogen (H₂)
Nickel catalyst (Ni)
Elevated temperature and pressure
This method is known as catalytic hydrogenation, where hydrogen molecules are activated on the surface of the metal catalyst.
Catalytic hydrogenation: A reduction reaction in which hydrogen is added to a molecule using a metal catalyst such as nickel.
The catalyst provides a surface where hydrogen atoms can react with the nitrile, breaking the triple bond and forming new C–H and N–H bonds.
Reaction Equation
The overall change can be represented by a general equation.
Reduction of a nitrile (R–C≡N) = R–CH₂NH₂
R = Alkyl group derived from the carbon chain
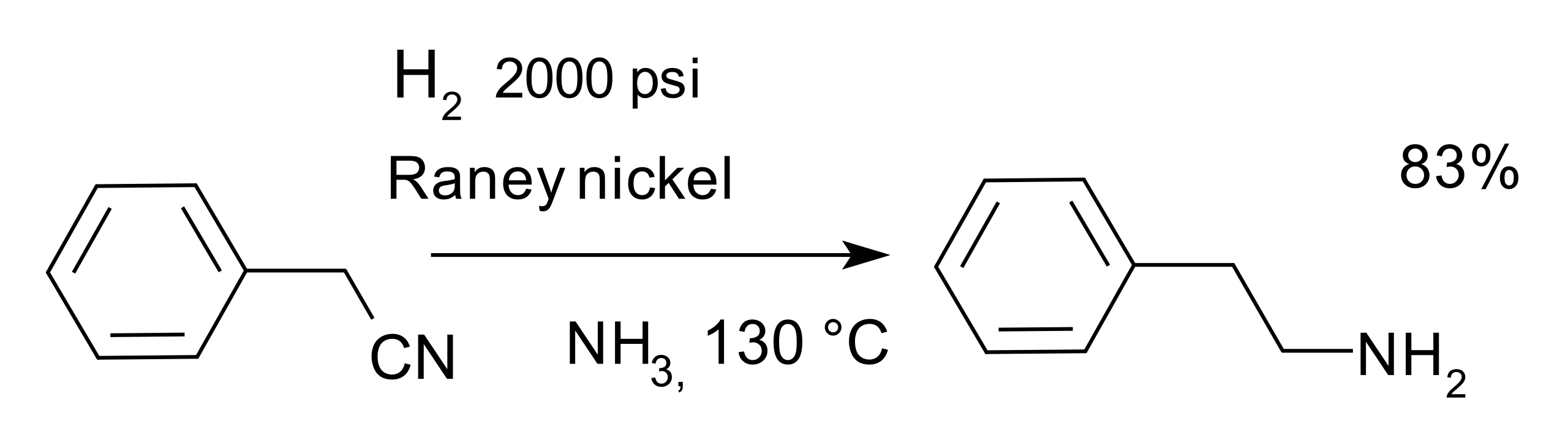
This diagram shows the overall hydrogenation of a nitrile to a primary amine. Two equivalents of hydrogen add across the C≡N bond, converting R–C≡N into R–CH₂NH₂. Source
This equation shows that no carbon atoms are lost during the reaction, reinforcing the importance of nitrile reduction for carbon-chain extension.
Reaction Pathway and Bond Changes
Although the detailed mechanism is not required at A-Level, students should understand the overall bond changes.
Key bond changes include:
Breaking the C≡N triple bond
Formation of C–N single bond
Addition of hydrogen to both carbon and nitrogen
The nitrile carbon is reduced from a high oxidation state to a lower one, consistent with the definition of reduction as gain of hydrogen.
Role in Organic Synthesis
Reducing nitriles to amines is particularly useful in multi-step synthetic routes. Nitriles themselves are commonly formed from haloalkanes via nucleophilic substitution with cyanide ions.
This sequence allows chemists to:
Extend a carbon chain by one carbon
Convert a haloalkane into an amine via two steps
Introduce nitrogen into an organic molecule in a controlled way
The amines produced can then undergo further reactions, including:
Formation of ammonium salts with acids
Reaction with acyl chlorides to form amides
Alkylation to produce secondary and tertiary amines
Comparison with Other Transformations
Unlike hydrolysis of nitriles, which forms carboxylic acids, reduction produces nitrogen-containing compounds. This distinction is important when designing synthetic routes, as the choice of reaction determines the functional group introduced.
Reduction:
Produces amines
Retains nitrogen in the molecule
This contrasts with hydrolysis, where nitrogen is removed as ammonium ions.
Exam Focus and Common Errors
Students should be careful to:
State the correct reagents (H₂/Ni)
Identify the product as a primary amine only
Avoid confusing reduction with hydrolysis or substitution
A frequent error is suggesting the formation of secondary amines or alcohols, which does not occur under these conditions.
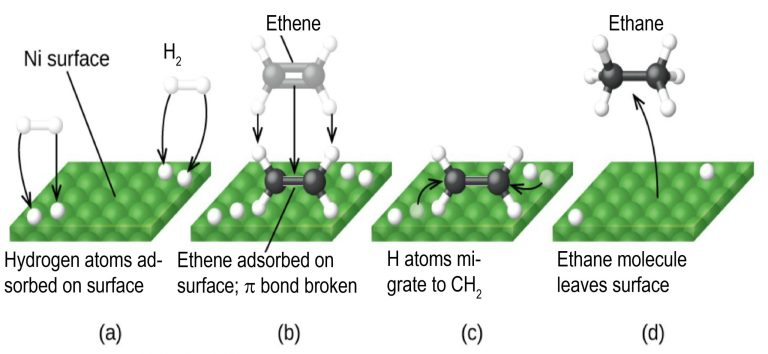
This figure illustrates the surface steps of heterogeneous catalytic hydrogenation on a nickel catalyst. The example shows ethene hydrogenation, but the same adsorption and hydrogen-transfer principles apply to H₂/Ni reduction of nitriles. Source
Understanding nitrile reduction strengthens overall competence in organic synthesis, particularly when analysing reaction sequences and predicting final products from given intermediates.
FAQ
Nitriles contain a very strong carbon–nitrogen triple bond, which has a high bond enthalpy and limited polarity.
This means:
More energy is needed to break the bond
A metal catalyst such as nickel is required to activate both the nitrile and hydrogen
Mild reducing agents that work for carbonyl compounds are ineffective for nitriles
As a result, nitrile reduction requires harsher conditions than many other organic reductions.
During hydrogenation, intermediates such as imines may form briefly on the metal surface.
However:
Imine intermediates remain adsorbed to the catalyst
Hydrogen continues to add rapidly under the reaction conditions
The fully reduced amine is more stable than the imine
This is why the reaction proceeds directly to a primary amine rather than stopping partway.
Molecular hydrogen (H₂) is relatively unreactive because of the strong H–H bond.
On a nickel surface:
The H–H bond is broken into hydrogen atoms
The nitrile is held close to these atoms
New C–H and N–H bonds can form efficiently
Without a catalyst, the activation energy for the reaction would be too high.
The carbon in the –C≡N group starts in a relatively high oxidation state due to its bonding to nitrogen.
During reduction:
Hydrogen is added to the carbon
The triple bond is replaced by single bonds
The oxidation state of carbon decreases
This change is consistent with the definition of reduction as gain of hydrogen.
Nitriles are particularly valuable because they can be made easily and converted predictably into amines.
Advantages include:
Simple formation from haloalkanes
Guaranteed formation of primary amines
Controlled extension of the carbon chain
This reliability makes nitrile reduction a common strategy in multi-stage organic synthesis.
Practice Questions
A nitrile can be converted into a primary amine during organic synthesis.
State the reagents and conditions required to reduce a nitrile to an amine.
(2 marks)
Hydrogen gas, H₂ (1 mark)
Nickel catalyst (accept Ni) with suitable conditions such as heat/pressure (1 mark)
Propanenitrile is used as an intermediate in a multi-step synthesis.
a) State the type of reaction that occurs when propanenitrile is converted into propylamine. (1 mark)
b) Give the reagents and conditions required for this reaction. (2 marks)
c) Explain why the product formed is a primary amine rather than a secondary or tertiary amine. (2 marks)
(5 marks)
a)
Reduction (1 mark)
b)
Hydrogen gas, H₂ (1 mark)
Nickel catalyst / catalytic hydrogenation conditions (1 mark)
c)
The carbon–nitrogen triple bond in the nitrile is reduced to give a –CH₂NH₂ group (1 mark)
No additional alkyl groups are added to the nitrogen, so only a primary amine can form (1 mark)
Maximum 5 marks

