OCR Specification focus:
‘Use C–C/N–C forming reactions to increase carbon chain length in synthesis.’
Introduction
Extending carbon chains is a central skill in advanced organic synthesis, enabling chemists to build more complex molecules by forming new carbon–carbon and nitrogen–carbon bonds.
Extending Carbon Chains in Organic Synthesis
Increasing the length of a carbon chain is essential when designing synthetic routes to larger, more functionalised molecules. OCR emphasises reactions that introduce new C–C or N–C bonds, allowing a small molecule to become the foundation for more advanced structures. These transformations are widely used in laboratory synthesis and industrial chemistry, giving access to pharmaceuticals, polymers, fragrances, and intermediates for multi-stage syntheses.
Importance of Carbon–Chain Extension
Creating new covalent bonds between carbon atoms significantly alters a molecule’s properties by increasing molecular weight, providing new reactive centres, or enabling branching. This process is often the first step in building a target molecule from simple starting materials. Many of these transformations use strong nucleophiles, reactive electrophiles, or reducing agents that modify functional groups after the initial chain extension.
Key C–C Bond-Forming Reactions
A range of reactions from across the specification contribute to carbon-chain extension. OCR focuses on those accessible at A-Level, with clear mechanistic pathways that illustrate nucleophilic attack, heterolytic bond cleavage, and subsequent functional group transformations.
Nucleophilic Substitution with Cyanide Ions
Haloalkanes undergo nucleophilic substitution when reacted with cyanide ions (CN⁻) in ethanol, producing nitriles, which contain an additional carbon atom compared with the starting haloalkane.
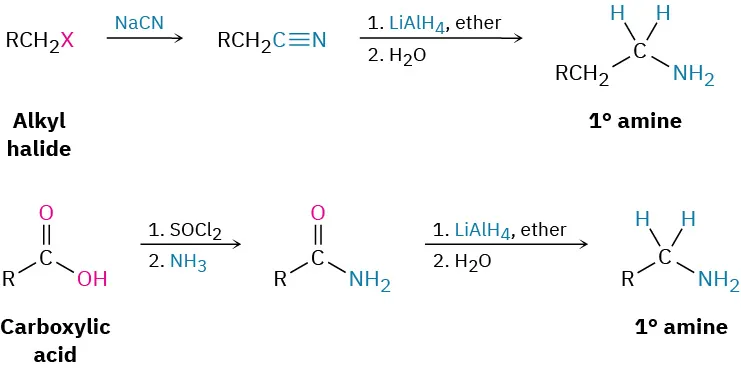
This scheme shows how an alkyl halide can be converted into a primary amine with one extra carbon by forming a nitrile intermediate using cyanide ions, followed by reduction. Source
Nitrile: An organic molecule containing the –C≡N functional group.
This reaction forms an N–C bond and effectively lengthens the carbon chain by one carbon. Although the detailed mechanism belongs to another subsubtopic, it is important here because nitriles produced by CN⁻ substitution serve as key intermediates in further transformations.
Nucleophilic Addition to Carbonyl Compounds
The addition of hydrogen cyanide (HCN) to aldehydes and ketones also extends carbon chains by one carbon.
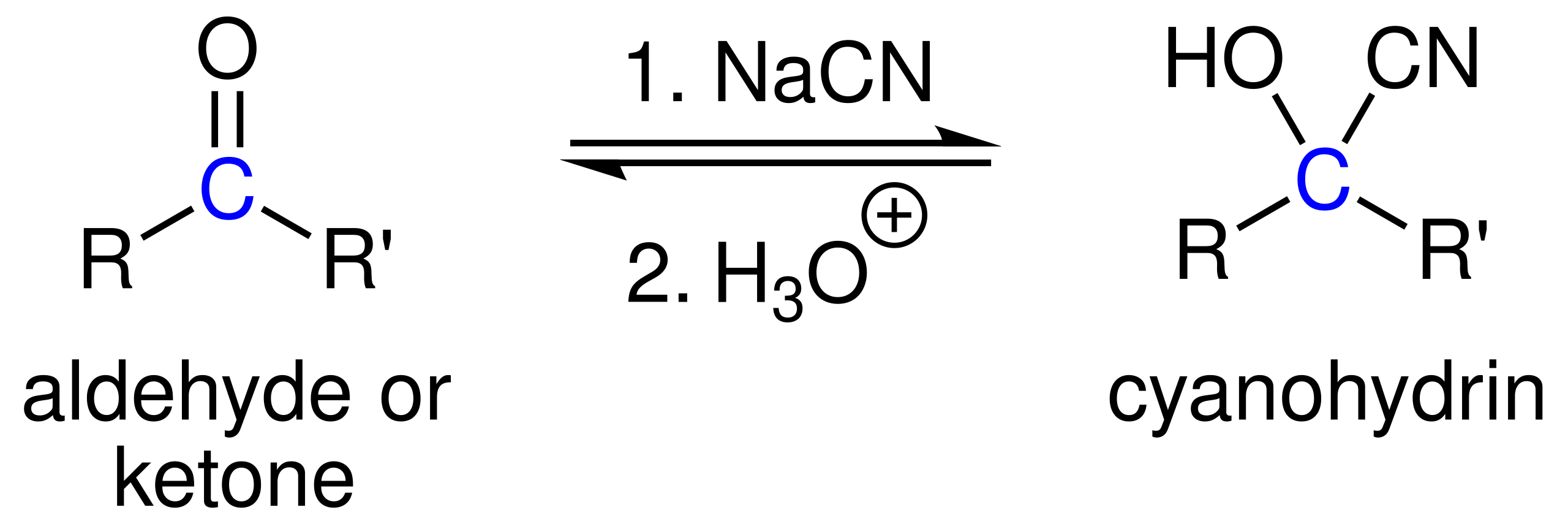
This diagram illustrates cyanohydrin formation, where hydrogen cyanide adds to a carbonyl compound to create a hydroxynitrile, extending the carbon chain by one atom. Source
The reaction proceeds by nucleophilic attack of CN⁻ on the partially positive carbonyl carbon, followed by protonation to yield hydroxynitriles. This pathway introduces both a new C–C bond and a reactive hydroxyl group at the same carbon, enabling versatile further modifications.
Hydroxynitrile: A molecule containing both an –OH group and a –C≡N group attached to the same carbon atom.
These functional groups make hydroxynitriles valuable intermediates, as they can be reduced, oxidised, or hydrolysed to generate a wide variety of compounds.
Functional Group Transformations After Chain Extension
Once the carbon chain has been extended using cyanide-based reactions, the resulting nitriles or hydroxynitriles often undergo further transformations to yield amines, carboxylic acids, or other important derivatives. These steps do not directly extend the chain further but convert the newly formed functional groups into those needed for a subsequent synthetic step.
Reduction of Nitriles
Nitriles can be reduced using hydrogen gas with a nickel catalyst to form primary amines, useful intermediates in pharmaceuticals, dyes, and other nitrogen-containing organic compounds.
Primary amine: An organic compound in which one alkyl or aryl group is attached to a nitrogen atom.
The product retains the additional carbon introduced during cyanide substitution, preserving the extended chain.
Acid Hydrolysis of Nitriles
Under hot aqueous acidic conditions, nitriles hydrolyse to form carboxylic acids, again conserving the chain extension.
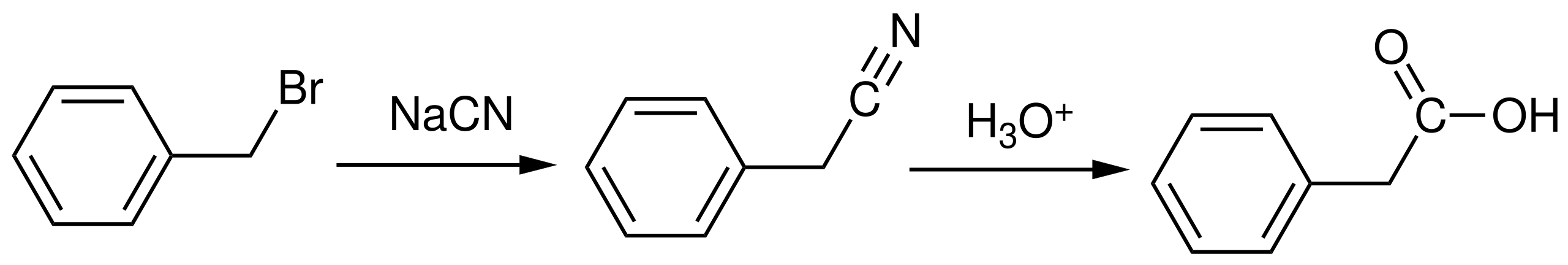
This reaction sequence shows how a nitrile formed during carbon-chain extension can be hydrolysed to a carboxylic acid, retaining the added carbon in the final product. Source
This is particularly valuable in multi-step synthesis because carboxylic acids can be transformed into esters, acyl chlorides, amides, and a variety of other functional groups.
Using Carbonyl Compounds in Chain Extension
Carbonyl compounds such as aldehydes and ketones play a major role in chain-extension strategies due to the electrophilic nature of the carbonyl carbon. Their susceptibility to nucleophilic attack makes them ideal starting materials for forming new C–C bonds.
Reactivity Considerations
Aldehydes are generally more reactive than ketones because of reduced steric hindrance and a greater partial positive charge on the carbonyl carbon. This difference influences reaction conditions and the likely yields of chain-extended products.
Designing Synthetic Routes Involving C–C Bond Formation
When planning a synthesis that requires carbon-chain extension, students must consider:
Suitable functional group interconversions before chain extension
Choice of reagents to form nitriles or hydroxynitriles
Reaction conditions, such as ethanolic medium or acidic conditions
Subsequent steps, including reduction or hydrolysis
Presence of other functional groups that may react competitively
Overall carbon count in the desired final molecule
Building effective synthetic pathways requires understanding both the mechanism of the C–C bond-forming step and the transformations available afterwards.
Typical Strategies for Carbon-Chain Extension
Below are the main strategies emphasised by OCR for extending carbon chains:
Reaction of haloalkanes with CN⁻ to form nitriles
Reaction of carbonyl compounds with HCN to form hydroxynitriles
Transformations of nitriles, including reduction and hydrolysis
Controlled reaction conditions to prevent unwanted side reactions
Use of carbonyl groups as electrophiles to generate new stereocentres in hydroxynitriles
Why Nitrile-Based Methods Are Central to A-Level Synthesis
Nitriles are key to chain extension because they:
introduce exactly one additional carbon
can be transformed into numerous functional groups
help build multi-stage routes in a controlled and predictable manner
appear commonly in exam synthesis pathways
Their versatility makes them essential tools for organic chemists and a central part of many OCR A-Level synthetic questions.
Carbon Count and Reaction Mapping
To successfully extend carbon chains in exam-style synthesis design, it is important to track how many carbons are introduced at each stage. For example:
Substitution with cyanide introduces +1 carbon
Nucleophilic addition of HCN introduces +1 carbon
Transformations following these steps maintain, but do not increase, carbon count
Students should mentally map each step, noting functional groups formed and those required for the next transformation.
Summary of Key Points for OCR
Reactions must form C–C or N–C bonds to qualify as chain extension
Cyanide-based mechanisms are the principal focus at A-Level
Resulting nitriles are crucial intermediates for further synthesis
Carbonyl compounds provide important electrophilic sites for extension
Understanding mechanisms helps predict products and plan routes
FAQ
Ethanolic conditions favour nucleophilic substitution over competing reactions.
In water, hydroxide ions are present and can cause unwanted hydrolysis of the haloalkane to an alcohol. Using ethanol reduces this competition, increasing the likelihood that CN⁻ attacks the carbon atom and forms the nitrile needed for chain extension.
Increasing carbon chain length generally alters intermolecular forces.
Longer chains typically show:
Higher boiling points due to increased London forces
Lower solubility in water
Greater non-polar character
These changes can influence purification methods and reaction conditions in synthesis.
Nitriles provide a stable, versatile functional group that can be selectively transformed.
They can be converted into:
Primary amines by reduction
Carboxylic acids by hydrolysis
This flexibility allows a single chain-extension step to feed into multiple synthetic pathways.
Cyanide compounds and hydrogen cyanide are highly toxic.
Strict controls are required, including:
Well-ventilated conditions
Careful handling and storage
Use of dilute solutions where possible
These precautions are essential in both educational and industrial laboratories.
Structural analysis focuses on carbon count and functional groups.
Evidence may include:
Increased molecular mass
Presence of a nitrile or derivative functional group
Changes in infrared absorption, such as a C≡N stretch
Together, these confirm successful formation of a longer carbon chain.
Practice Questions
A student reacts 1-bromopropane with ethanolic potassium cyanide.
a) Name the type of reaction that occurs.
b) State how the number of carbon atoms in the organic product compares with the starting haloalkane.
(2 marks)
a) Nucleophilic substitution
1 mark
b) The product contains one more carbon atom than the haloalkane
1 mark
Propanal can be converted into a carboxylic acid with a longer carbon chain using reactions studied in this specification.
a) Identify a reagent that reacts with propanal to increase the length of its carbon chain.
b) Name the type of reaction that occurs in this step.
c) Name the functional group present in the product of this reaction.
d) Describe how this product can be converted into a carboxylic acid with the longer carbon chain.
e) Explain why this sequence of reactions is useful in multi-stage organic synthesis.
(5 marks)
a) Hydrogen cyanide / HCN (or cyanide ions with suitable source)
1 mark
b) Nucleophilic addition
1 mark
c) Nitrile (accept hydroxynitrile / cyanohydrin)
1 mark
d) Acid hydrolysis of the nitrile using hot aqueous acid to form a carboxylic acid
2 marks
Hydrolysis mentioned
Acidic conditions stated
e) Allows controlled increase in carbon chain length and conversion into different functional groups / provides versatile intermediates for further reactions
1 mark

