OCR Specification focus:
‘Interpret gas chromatograms using retention times to identify mixture components.’
Gas chromatography (GC) is a key analytical technique used to separate, detect, and identify volatile components in a mixture. Understanding retention times is essential for interpreting chromatograms accurately and linking each peak to a specific compound.
Introduction
Gas chromatography separates volatile substances as they travel through a column, producing measurable retention times that help identify mixture components clearly and reliably.
Understanding Gas Chromatography
GC operates by distributing components between a mobile phase (an inert carrier gas such as helium or nitrogen) and a stationary phase (a liquid or polymer coated onto the inside of a long column). As compounds pass through the column, they move at different rates depending on their interactions with the stationary phase.
A gas chromatograph has an injector, a long column in a temperature-controlled oven, and a detector linked to a computer.
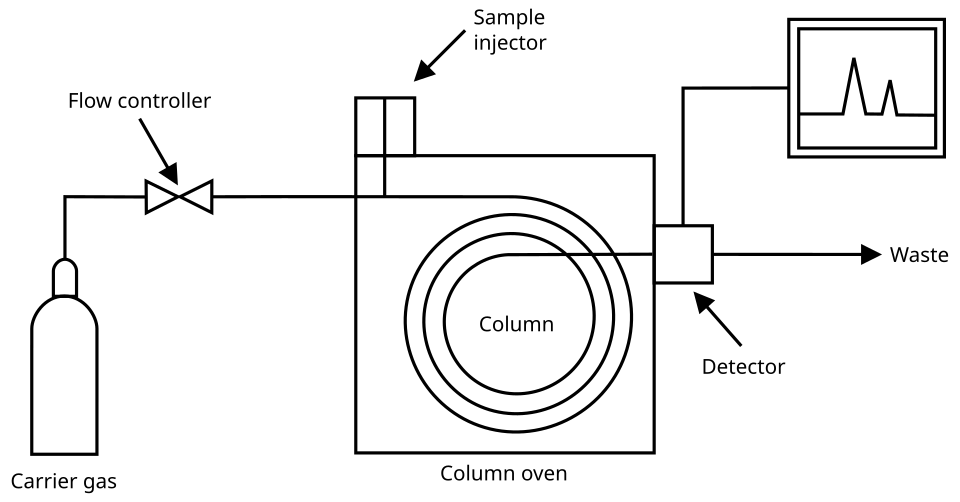
This diagram shows the main components of a gas chromatograph: carrier gas supply, injector, column in an oven, and detector. Retention times depend on column properties, oven temperature, and carrier gas flow. Source
What Retention Time Represents
The retention time is the time taken for a compound to pass from injection to detection. It is the fundamental measure used to interpret gas chromatograms in A-Level Chemistry.
Retention Time: The time taken for a compound to travel from the point of injection to the detector in a gas chromatograph.
Retention time provides a characteristic fingerprint for a compound under fixed operating conditions. Students must be familiar with how these values are used to identify components in mixtures.
After a definition has been established, it is essential to appreciate that retention times are only meaningful when the GC conditions are controlled and reproducible, such as temperature, flow rate, and column characteristics.
Factors Affecting Retention Time
Several controlled variables influence how long a compound remains in the column.
Key Factors
Boiling point
• Higher-boiling substances tend to have longer retention times because they spend more time in the liquid stationary phase.Polarity and stationary-phase interactions
• Stronger attraction to the stationary phase increases retention time.Column temperature
• Higher temperatures reduce retention times by increasing compound volatility.Carrier-gas flow rate
• Faster flow reduces retention time by shortening the travel duration through the column.Column length and internal diameter
• Longer columns provide greater separation but increase overall retention times.
Each of these factors must remain consistent when comparing chromatograms, as changes can shift retention times and alter the appearance of peaks.
Interpreting Retention Times
The OCR specification requires students to interpret gas chromatograms using retention times to identify mixture components. Identification relies on comparing measured retention times with reference values from known standards run under identical conditions.
How Peaks Represent Components
Each peak in a chromatogram corresponds to a compound that elutes from the column. The position of the peak along the time axis indicates its retention time.
A gas chromatogram plots detector response against time; each peak corresponds to a component leaving the column.
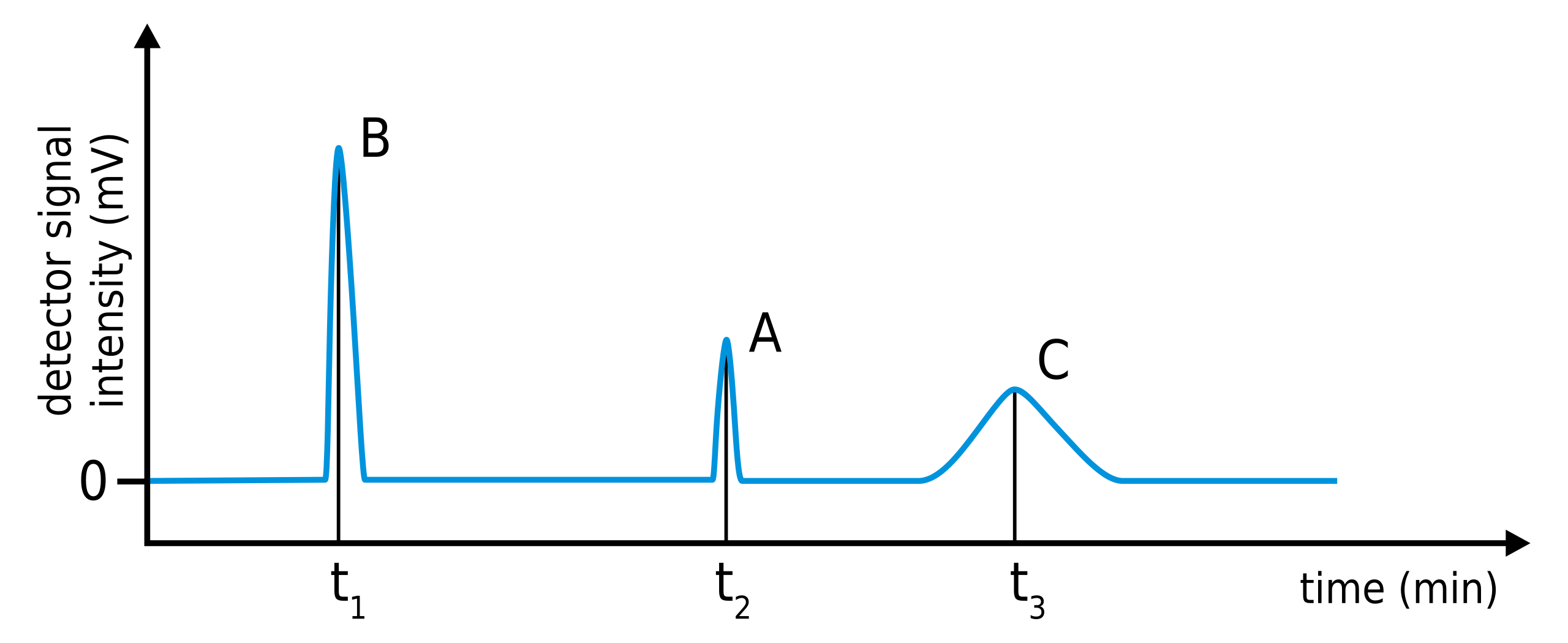
This chromatogram shows detector response plotted against time. The retention time of each component is read from the time axis at the position of its peak, allowing identification by comparison with known standards. Source
Using Retention Times for Identification
To identify components reliably, retention times should be matched to previously recorded values:
Run reference standards under the same GC conditions.
Record their retention times.
Compare the retention times of unknown peaks with those of the standards.
A match suggests the identity of the unknown compound.
This comparison works because retention time is reproducible when all GC parameters are unchanged.
After establishing this principle, it is useful to recognise that slight variations may still occur. Therefore, high-precision chromatographic systems often include internal standards to maintain accuracy.
Limitations of Retention-Time Identification
Although retention times are powerful indicators, they do not provide absolute proof of identity. Two different compounds may share similar retention times if they have comparable properties.
Common Limitations
Co-elution occurs when two compounds emerge at nearly the same time, producing overlapping peaks.
Instrument drift may alter retention times across different runs.
Structural similarity between compounds with similar polarity and boiling points may produce indistinguishable retention times.
Changes in column ageing can affect interactions with the stationary phase.
Students should be aware that GC is often paired with other analytical methods, such as mass spectrometry, when definitive identification is required.
Practical Use of Retention Times
Retention-time data allow chemists to examine mixture composition quickly.
Applications in Practice
Quality control in chemical manufacturing, where known compounds must fall within expected retention-time windows.
Environmental testing, such as detecting pollutants in air or water.
Forensic analysis of volatile organic compounds.
Pharmaceutical development, ensuring purity and identifying trace contaminants.
These examples highlight the broad utility of GC retention times in professional laboratories.
After discussing applications, it becomes clear that retention-time interpretation is a universal skill across chemical disciplines, making it essential for A-Level study.
Ensuring Accurate Retention-Time Measurements
For retention times to be meaningful, the GC system must operate under carefully controlled conditions.
To identify a component, compare its retention time with a chromatogram from a known standard recorded using the same column and identical operating conditions.
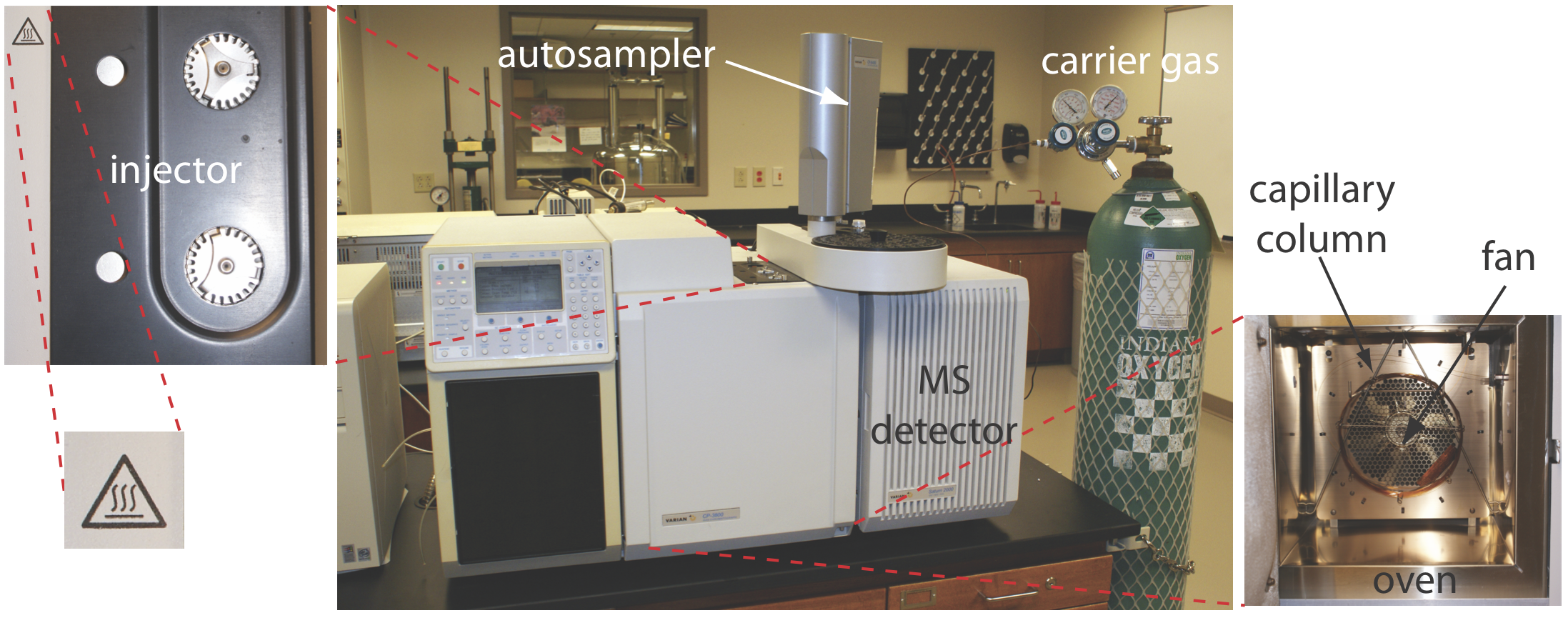
This photograph shows a typical laboratory gas chromatograph with a heated injector and column oven. The image also includes an autosampler and mass spectrometer detector, which extend beyond the syllabus focus on retention times alone. Source
Good Practice
Maintain constant oven temperature throughout the run.
Use a consistent carrier-gas flow rate.
Ensure the stationary phase and column are appropriate for the sample type.
Regularly calibrate the instrument using standards.
Inject consistent sample volumes to avoid peak-shape distortion.
Small procedural changes can significantly affect retention times, so precision is vital.
Importance of Consistency
Reproducible conditions allow students and chemists to compare chromatograms across different analyses and confidently identify mixture components using retention-time data.
These study notes provide all the core knowledge required by the specification for interpreting gas chromatograms using retention times at OCR A-Level Chemistry standard.
FAQ
Retention times depend strongly on experimental conditions rather than being fixed physical constants. Changes in oven temperature, carrier gas flow rate, or column type will alter how long a compound remains in the column.
Even small adjustments can shift retention times enough to cause misidentification. For this reason, unknown samples must always be compared with reference standards analysed using the same column and identical operating conditions.
Column polarity influences how strongly compounds interact with the stationary phase.
Non-polar columns retain non-polar compounds more strongly.
Polar columns increase retention times for polar compounds due to stronger intermolecular attractions.
Choosing a suitable column improves separation and helps produce retention times that clearly distinguish between mixture components.
Raising the oven temperature increases the kinetic energy and volatility of the components. This reduces the time compounds spend dissolved in the stationary phase.
As a result, substances travel through the column more quickly, leading to shorter retention times. Temperature control is therefore critical for reproducible chromatograms.
Retention time reproducibility refers to obtaining the same retention time for a compound across repeated runs under identical conditions.
Good reproducibility is important because it ensures that small variations in chromatograms reflect real differences in samples rather than instrument instability. This increases confidence when identifying components by comparison with standards.
Different compounds can show similar retention times if they have comparable boiling points and similar interactions with the stationary phase.
This is more likely when compounds have related structures or functional groups. In such cases, retention time alone may be insufficient for definite identification, and additional analytical techniques may be required.
Practice Questions
A mixture is analysed using gas chromatography. Explain what is meant by the term retention time and state how it is used to help identify a component in the mixture.
(2 marks)
Award one mark for each of the following points:
Correct definition of retention time as the time taken for a compound to travel from injection to detection. (1 mark)
Correct explanation that retention time is compared with known or reference values (standards) run under the same conditions to identify a component. (1 mark)
A student analyses a liquid mixture using gas chromatography and obtains a chromatogram with three distinct peaks.
a) Explain why different components in the mixture have different retention times. (3 marks)
b) Describe how the student could use retention times to identify one of the components in the mixture. (2 marks)
(5 marks)
a) Explanation of different retention times (3 marks)
Award up to three marks from:
Different components interact to different extents with the stationary phase. (1 mark)
Compounds with higher boiling points or stronger attractions to the stationary phase spend longer in the column. (1 mark)
Differences in volatility or polarity cause components to move through the column at different speeds. (1 mark)
b) Identification using retention times (2 marks)
Award one mark for each of the following points:
Retention time of the unknown peak is measured from the chromatogram. (1 mark)
Retention time is compared with retention times of known standards obtained under identical GC conditions to identify the component. (1 mark)

