OCR Specification focus:
‘Use 2,4-DNP for C=O; Tollens’ for aldehydes; acidified dichromate distinguishes alcohols and aldehydes.’
This subsubtopic explores essential test-tube reactions used to distinguish carbonyl compounds, aldehydes, and alcohols, forming a core part of qualitative organic analysis in A-Level Chemistry.
Test-Tube Reactions for Carbonyl Compounds
Understanding the behaviour of carbonyl-containing compounds is central to qualitative identification, especially through their reactions with 2,4-dinitrophenylhydrazine (2,4-DNP).
Detecting the Carbonyl Group with 2,4-DNP
The reagent 2,4-DNP is used to identify the presence of a C=O functional group in aldehydes and ketones. When added to a compound containing a carbonyl group, 2,4-DNP undergoes a characteristic condensation reaction to form an orange-yellow precipitate called a 2,4-dinitrophenylhydrazone derivative, confirming the presence of the carbonyl functionality.
The reaction does not occur with alcohols, carboxylic acids, or esters, making it highly selective within this context.
Carbonyl group: A functional group consisting of a carbon atom double-bonded to an oxygen atom (C=O).
A positive 2,4-DNP test provides strong evidence for a carbonyl compound, but the test alone cannot determine whether the compound is an aldehyde or a ketone. Further oxidation-based tests are required for that distinction.
Key Features of the 2,4-DNP Reaction
Forms a solid orange-yellow precipitate, confirming a carbonyl group
Works reliably with both aldehydes and ketones
Does not react with alcohols or other non-carbonyl compounds
Useful for preliminary identification prior to more selective testing
Add 2,4-DNPH (Brady’s reagent); a yellow/orange/red precipitate indicates a carbonyl (C=O) group.
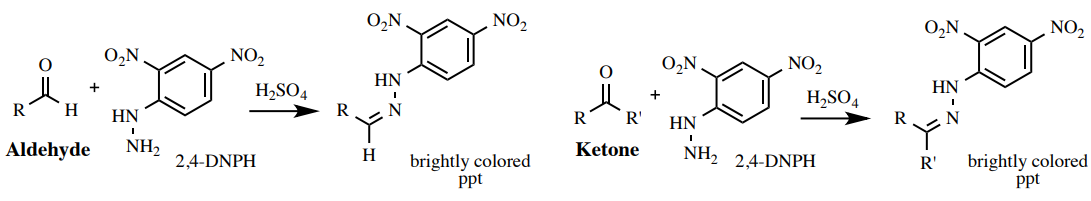
This diagram shows aldehydes and ketones reacting with 2,4-DNPH to form a brightly coloured 2,4-dinitrophenylhydrazone precipitate, confirming the presence of a carbonyl group. Source
Distinguishing Aldehydes from Ketones
After confirming the presence of a carbonyl group, the next step is to determine whether the compound is an aldehyde or a ketone. Aldehydes are generally more easily oxidised than ketones, allowing selective reagents such as Tollens’ reagent and acidified potassium dichromate(VI) to differentiate between them.
Tollens’ Reagent: Identifying Aldehydes
Tollens’ reagent is a mild oxidising agent containing the diamminesilver(I) complex. It is specifically used to distinguish aldehydes from ketones because aldehydes can be oxidised to carboxylates under these gentle conditions, while ketones resist oxidation.
Tollens’ reagent: An aqueous solution containing [Ag(NH₃)₂]⁺, used to oxidise aldehydes to carboxylates while reducing silver(I) ions to metallic silver.
When an aldehyde is warmed with Tollens’ reagent, a characteristic silver mirror forms on the inner surface of the test tube. This arises due to the reduction of Ag⁺ to silver metal. Ketones do not react, providing a clear negative result.
Tollens’ reagent gives a silver mirror with aldehydes, but not with ketones.

A positive Tollens’ test produces a silver mirror as silver(I) ions are reduced to metallic silver, indicating the presence of an aldehyde. Source
Oxidation Behaviour with Tollens’
Aldehydes are oxidised to carboxylate ions
Silver(I) ions are reduced to metallic silver
Ketones do not undergo oxidation, giving no visible change
The test is highly selective and widely used in qualitative analysis
A brief interval between definition blocks is important to contextualise how Tollens’ reagent fits into the broader analytical sequence, as it is typically used after 2,4-DNP has confirmed the presence of a carbonyl group.
Distinguishing Alcohols and Aldehydes with Acidified Dichromate
Another key reagent for this subsubtopic is acidified potassium dichromate(VI), a stronger oxidising agent capable of oxidising both alcohols and aldehydes. Its colour change makes it especially useful in practical laboratory identification.
Acidified Potassium Dichromate(VI) as an Oxidising Agent
When heated with aldehydes or primary and secondary alcohols, acidified dichromate(VI) undergoes reduction from orange Cr₂O₇²⁻ to green Cr³⁺. This visible transformation provides an immediate indication that oxidation has taken place.
Tertiary alcohols do not react, allowing differentiation between alcohol classes.
Oxidising agent: A substance that causes oxidation of another species by accepting electrons and being reduced in the process.
Because aldehydes are easily oxidised to carboxylic acids, they give a positive test with acidified dichromate(VI). Ketones, however, are resistant to oxidation and do not trigger the colour change. This behaviour allows identification of carbonyl types using a different mechanism from Tollens’ reagent.
With acidified dichromate(VI), oxidation is indicated by the reagent changing from orange to green.
This diagram illustrates the reduction of chromium(VI) to chromium(III), corresponding to the orange-to-green colour change observed when aldehydes or oxidisable alcohols react with acidified dichromate(VI). Source
Using Dichromate(VI) in Practice
Primary alcohols oxidise to aldehydes and further to carboxylic acids
Secondary alcohols oxidise to ketones
Tertiary alcohols show no reaction, remaining orange
Aldehydes cause the reagent to turn green due to reduction
Ketones produce no visible change
Useful both for identifying alcohol classes and distinguishing aldehydes from ketones
Between tests, the relative oxidation ease helps students understand why aldehydes react with both Tollens’ and dichromate(VI), while ketones resist both reagents.
Summary of Key Test-Tube Reactions in This Subsubtopic
Although this section avoids providing a conclusion, it is important within the notes to consolidate the essential qualitative distinctions described above:
Essential Points
2,4-DNP confirms the presence of a carbonyl group via an orange-yellow precipitate
Tollens’ reagent identifies aldehydes through formation of a silver mirror
Acidified dichromate(VI) distinguishes alcohol classes and confirms aldehydes by an orange-to-green colour change
Ketones provide negative results with both Tollens’ reagent and dichromate(VI), aiding clear differentiation
FAQ
The colour variation arises from differences in the molecular structure of the 2,4-dinitrophenylhydrazone formed.
Conjugation length, substituent effects, and molecular symmetry influence how light is absorbed, leading to shades ranging from yellow to deep orange or red.
These colour differences are not used for identification at A-Level, but they can indicate structural variation in carbonyl compounds.
Tollens’ reagent is unstable and decomposes on standing.
If left unused, it can form silver nitride or silver fulminate residues, which are potentially explosive when dry.
Preparing the reagent immediately before testing ensures:
Reliable oxidation of aldehydes
Safe laboratory practice
Clear formation of the silver mirror
Ketones lack a hydrogen atom bonded to the carbonyl carbon, making them resistant to mild oxidation.
Oxidising a ketone would require breaking a carbon–carbon bond, which Tollens’ reagent is not strong enough to achieve.
As a result, ketones remain unchanged, providing a negative result.
Heating increases the rate of oxidation by providing energy for bond breaking.
Without heating:
Primary and secondary alcohols may react very slowly
Colour change may be incomplete or difficult to observe
Gentle heating ensures a clear orange-to-green transition within a practical timescale.
Both aldehydes and primary alcohols are readily oxidised by dichromate(VI), producing the same orange-to-green colour change.
Because the same visual result occurs:
The reagent confirms oxidation only
Additional tests, such as Tollens’ reagent, are needed for clear identification
Practice Questions
A student adds 2,4-dinitrophenylhydrazine (2,4-DNP) to an unknown organic compound and observes an orange precipitate.
a) What functional group is indicated by this result?
b) State one class of organic compound that would not give this result.
(2 marks)
a) Carbonyl (C=O) group
1 mark
b) Any one correct answer:
Alcohol
Carboxylic acid
Ester
Alkane
1 mark
A student is given three unlabelled organic compounds: A, B and C.
Compound A gives an orange precipitate with 2,4-DNP and a silver mirror with Tollens’ reagent.
Compound B gives an orange precipitate with 2,4-DNP but no visible change with Tollens’ reagent.
Compound C turns acidified potassium dichromate(VI) solution from orange to green but gives no reaction with 2,4-DNP.
Using this information:
a) Identify the type of organic compound A, B and C.
b) Explain the observations for compound C when tested with acidified potassium dichromate(VI).
(5 marks)
a) Identification of compounds (3 marks total)
Compound A is an aldehyde
1 mark
Compound B is a ketone
1 mark
Compound C is an alcohol (primary or secondary)
1 mark
b) Explanation for compound C (2 marks total)
Acidified potassium dichromate(VI) is reduced from Cr2O7²⁻ (orange) to Cr³⁺ (green)
1 mark
The alcohol is oxidised, showing that it is not a tertiary alcohol
1 mark
Maximum 5 marks

