OCR Specification focus:
‘Phenols are weak acids that do not form bubbles with carbonates; carboxylic acids react with CO3²⁻.’
Phenols and carboxylic acids show characteristic test-tube behaviours that allow their differentiation through simple reactions, especially acid–base interactions and observable gas evolution.
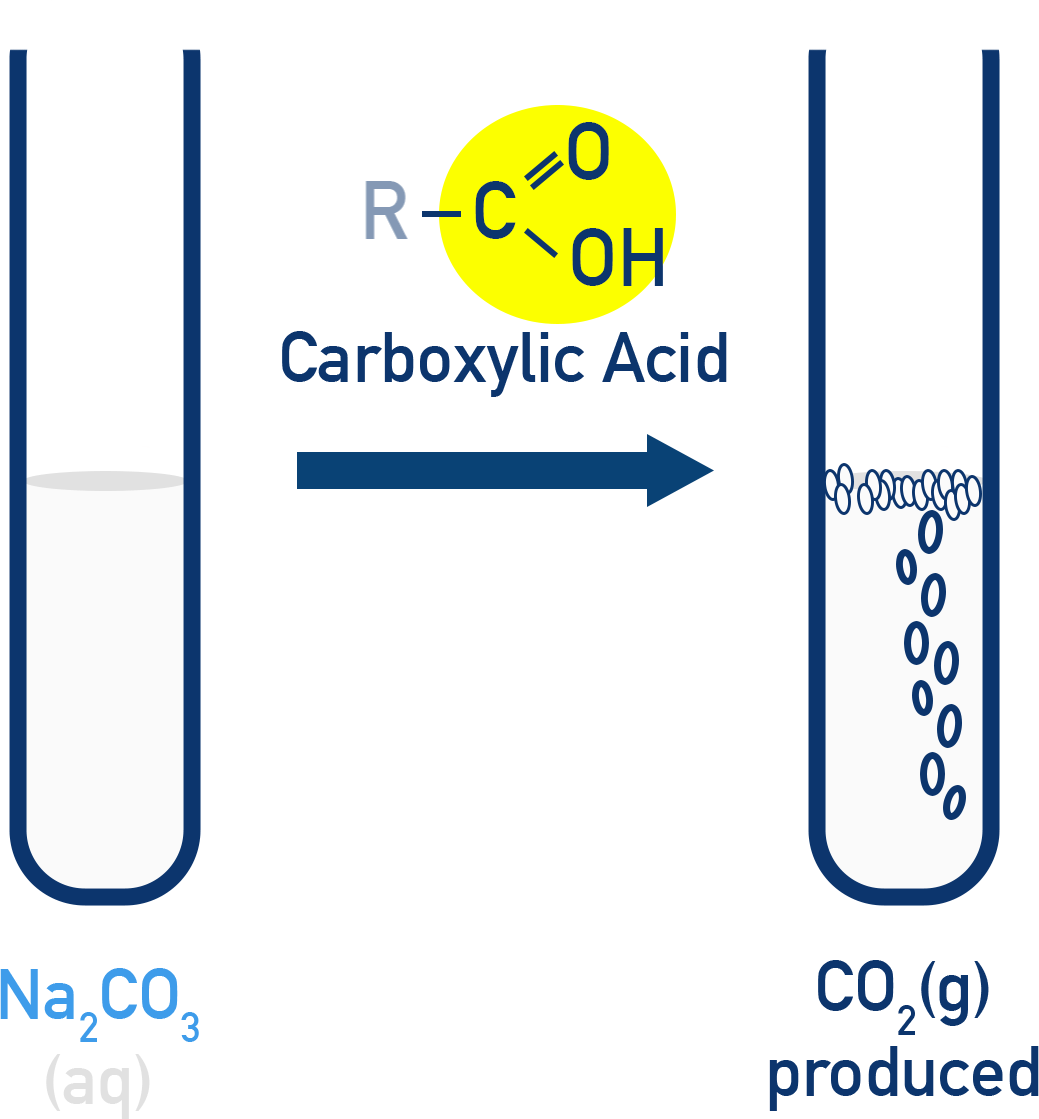
This diagram illustrates the carbonate test for a carboxylic acid, where reaction with aqueous sodium carbonate produces carbon dioxide gas, observed as effervescence. The release of CO₂ is the key qualitative observation used to identify a –COOH group. Source
Test-Tube Identification of Phenols and Carboxylic Acids
Phenols and carboxylic acids are both acidic organic compounds, yet their reactivity in qualitative tests differs significantly. Understanding these differences is essential for recognising functional groups in laboratory analysis and for interpreting organic reaction behaviour.
Comparing Acidity and Ion Formation
Phenols contain an –OH group directly attached to an aromatic ring, giving them distinctive reactivity.
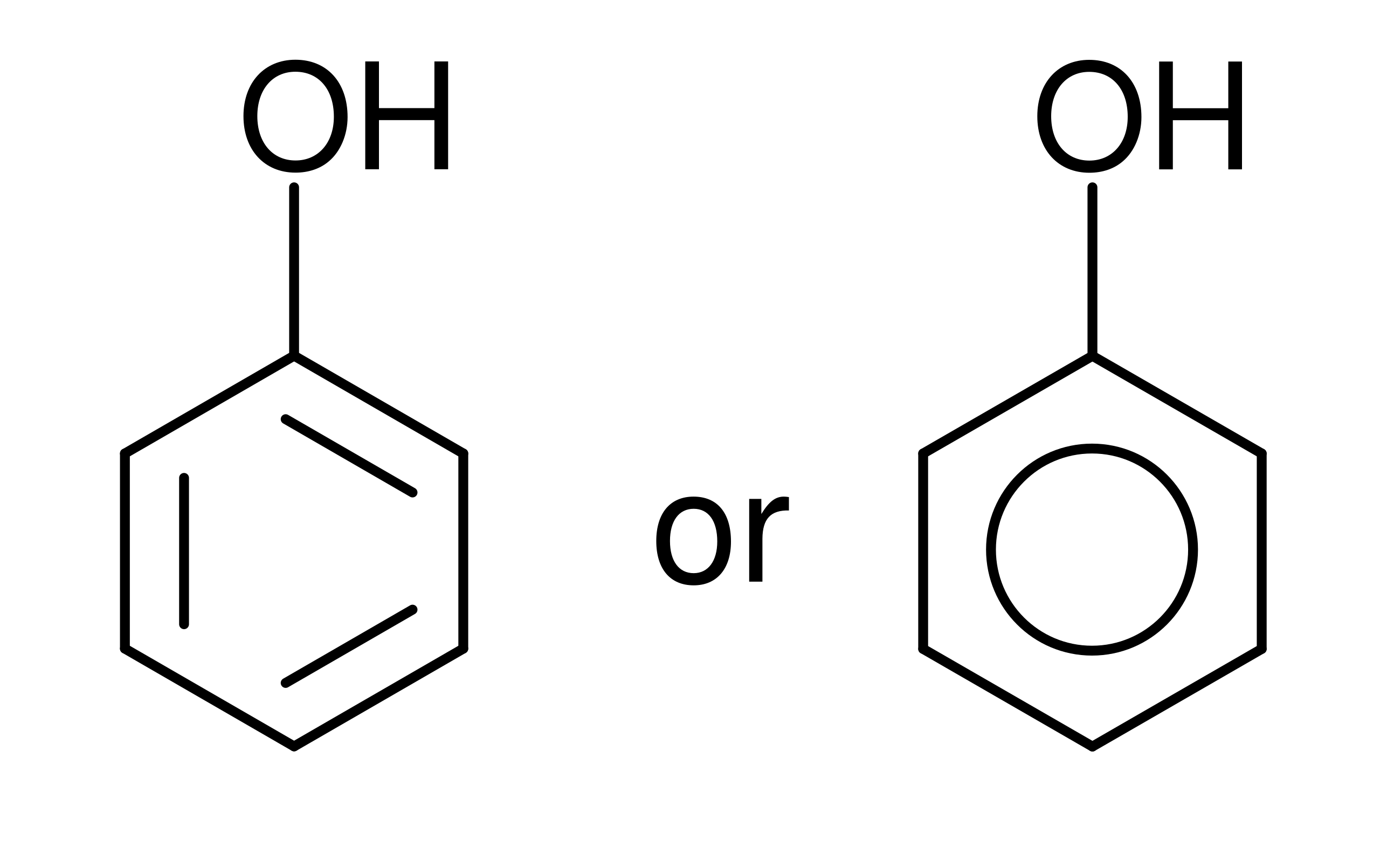
This structural formula shows phenol, with an –OH group directly bonded to an aromatic benzene ring. This bonding arrangement distinguishes phenols from alcohols and influences their acidity and test-tube reactions. Source
Carboxylic acids contain the –COOH functional group. Their differing abilities to donate protons influence their reactions with bases and carbonates.
Weak Acid: A substance that partially dissociates in aqueous solution, producing a limited concentration of hydrogen ions.
Because phenols are weak acids, they only partially form phenoxide ions in aqueous solution and cannot neutralise weak bases such as carbonates. This behaviour directly links to their laboratory test outcomes.
A brief examination of structural differences highlights why carboxylic acids are sufficiently acidic to react with carbonates, generating carbon dioxide gas, whereas phenols are not.
Reaction of Phenols with Carbonates
A key diagnostic test is the addition of solid sodium carbonate or sodium hydrogen carbonate to the organic compound.
Phenols do not react with carbonates.
No effervescence (bubbling) is observed.
The absence of carbon dioxide release is a primary indicator that the unknown compound may be a phenol rather than a carboxylic acid.
This lack of reactivity results from phenols being too weakly acidic to donate protons to carbonate ions.
Reaction of Carboxylic Acids with Carbonates
Carboxylic acids readily undergo acid–base reactions due to their greater acidity.
Carboxylic acids react vigorously with carbonates.
Effervescence occurs due to the release of carbon dioxide gas.
The reaction proceeds even in dilute solutions.
Acid–Carbonate Reaction (General) = Acid + Carbonate → Salt + Water + Carbon Dioxide
CO₂ = Carbon dioxide gas released during effervescence
In test-tube analysis, the appearance of bubbles is the crucial qualitative observation, signalling the presence of a carboxylic acid functional group.
A sentence placed here ensures no consecutive equation or definition blocks are adjacent.
Formation of Salts in Acid–Base Reactions
Carboxylic acids form carboxylate salts (e.g., ethanoates, propanoates) when reacting with metal carbonates or hydroxides. Phenols form phenoxide ions only when treated with strong bases such as sodium hydroxide, not with carbonates.
Phenoxide Ion: The anion formed when a phenol loses a proton from its hydroxyl group, typically in reaction with strong bases.
Unlike carboxylates, phenoxide salts are only produced in the presence of hydroxides due to phenols’ lower acidity.
Testing with Strong Bases
The different behaviours of phenols and carboxylic acids become clearer when strong bases are used:
Phenols react with sodium hydroxide, forming soluble sodium phenoxide.
Carboxylic acids also react with sodium hydroxide, producing carboxylate salts and water.
Both dissolve on addition of strong base, meaning this test cannot distinguish them reliably.
The carbonate test remains the key differentiation method.
Role of Delocalisation and Acidity Strength
The acidity of carboxylic acids arises from stabilisation of the carboxylate ion by resonance delocalisation between both oxygens. Phenols also exhibit some delocalisation when forming phenoxide ions, but the stabilisation is much weaker, reducing their acidic strength.
This difference in stabilisation energy explains why only carboxylic acids are strong enough to release carbon dioxide from carbonates.
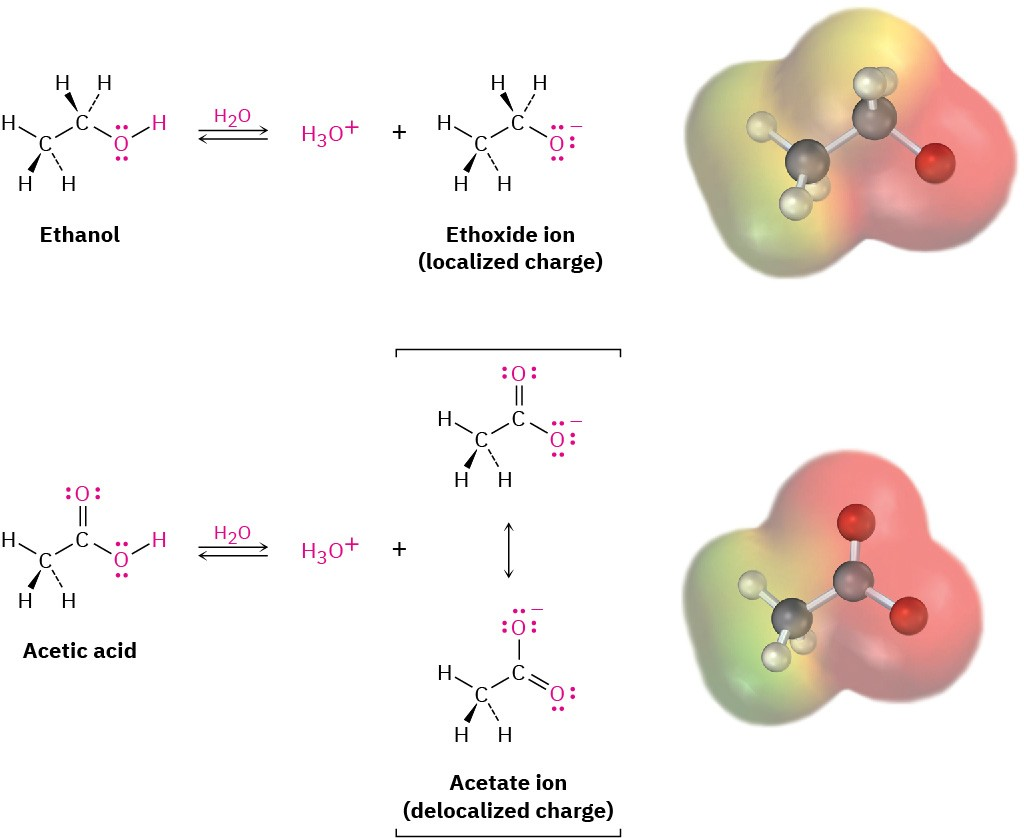
This figure compares a localised negative charge in an alkoxide ion with the delocalised charge in a carboxylate ion. It also includes alcohol–acid comparisons, which go beyond the OCR test-tube requirement but help explain why carboxylic acids are stronger acids than phenols. Source
Practical Considerations in Test-Tube Procedures
When carrying out these tests in a laboratory setting:
Use small quantities of reagents to observe changes clearly.
Add carbonate powders slowly to prevent vigorous reactions with strong acids.
Warmth is not required; reactions occur at room temperature.
Always compare the compound’s behaviour with a known reference sample.
Visual cues are central to these qualitative tests.
Additional Observations with Phenols
Although they do not react with carbonates, phenols exhibit other distinctive behaviours that help support their identification:
No effervescence on contact with carbonate solutions.
Slight acidity, sufficient to react with strong bases but not weak ones.
Insolubility in water unless converted into phenoxide ions.
These properties help differentiate phenols from alcohols and carboxylic acids in broader qualitative schemes.
Additional Observations with Carboxylic Acids
Carboxylic acids show predictable acid reactions:
Effervescence with carbonates due to CO₂ release.
Characteristic vinegar-like odours for many lower carboxylic acids.
Formation of salts with both strong and weak bases.
Their behaviour is consistent and easily recognisable in a laboratory context.
Summary of Key Distinguishing Features
For quick reference in test-tube identification:
Phenols:
Weak acids
Do not react with carbonates
No effervescence
React only with strong bases
Carboxylic acids:
Stronger acids
React with carbonates
Effervescence due to CO₂
React with both weak and strong bases
The contrast in carbonate reactivity forms the basis of the OCR specification requirement for distinguishing phenols from carboxylic acids during qualitative analysis.
FAQ
Phenols are acidic because they can donate a proton, but their acidity is too weak to react with hydrogen carbonate ions.
Hydrogen carbonate is a weak base and only reacts with acids strong enough to transfer protons readily. Phenols do not provide a sufficient concentration of hydrogen ions in aqueous solution to produce carbon dioxide gas, so no visible reaction occurs.
Effervescence provides clear visual evidence of a gas being released during a reaction.
In the carbonate test, bubbling indicates the formation of carbon dioxide. This confirms that an acid–carbonate reaction is occurring, which is characteristic of carboxylic acids and not phenols. The presence or absence of effervescence allows rapid functional group identification.
Sodium hydrogen carbonate reacts more gently with acids than sodium carbonate.
This makes observations easier to control and safer in a laboratory setting. Effervescence occurs at a slower rate, allowing clearer distinction between a positive reaction (carboxylic acid) and no reaction (phenol).
Yes, phenols and alcohols can show similar behaviour in some simple tests.
Both do not react with carbonates and do not produce effervescence. However, phenols are weakly acidic and react with strong bases, whereas most alcohols do not, allowing further differentiation if required.
The carbonate test relies on a clear chemical difference in acid strength.
Carboxylic acids consistently release carbon dioxide when reacting with carbonates, producing visible bubbles. Phenols never produce this reaction under the same conditions. This sharp contrast makes the test reliable and easy to interpret at A-level.
Practice Questions
A student adds solid sodium carbonate to separate samples of a phenol and a carboxylic acid in test tubes.
a) State the observation seen with the carboxylic acid.
b) State the observation seen with the phenol.
(2 marks)
a)
Effervescence / bubbling observed (1 mark)
Gas produced is carbon dioxide (1 mark)
b)
No effervescence / no bubbles observed (1 mark)
No reaction with sodium carbonate (1 mark)
(Any two correct points, one from each part, award 1 mark each)
A student is given an unknown organic compound that contains either a phenol group or a carboxylic acid group.
The student adds aqueous sodium hydrogen carbonate to the compound.
a) Describe the observation that would indicate the compound is a carboxylic acid.
b) Write the ionic equation for the reaction that occurs if the compound is a carboxylic acid.
c) Explain why no visible reaction is observed if the compound is a phenol.
(5 marks)
a)
Effervescence observed (1 mark)
Carbon dioxide gas released (1 mark)
b)
H⁺(aq) + HCO₃⁻(aq) → CO₂(g) + H₂O(l) (1 mark)
c)
Phenols are weak acids (1 mark)
They do not donate protons readily to hydrogen carbonate / carbonate ions (1 mark)
Therefore carbon dioxide is not produced / no effervescence observed (1 mark)
(Maximum 5 marks)

