OCR Specification focus:
‘Use the n + 1 rule for spin–spin splitting; recognise aromatic proton chemical shifts.’
Proton NMR splitting patterns and aromatic proton signals help identify structural features in organic molecules by revealing neighbouring proton numbers and characteristic aromatic chemical-shift ranges.
Proton NMR Splitting: Core Principles
Proton NMR spectroscopy provides detailed information about proton environments, neighbouring groups, and structural patterns in organic molecules. A key aspect of interpreting spectra is understanding spin–spin splitting, which shows how many protons are adjacent to a particular hydrogen.
The Origin of Spin–Spin Coupling
Spin–spin coupling occurs when non-equivalent neighbouring protons influence one another’s magnetic environment, causing a signal to appear as multiple peaks rather than a single resonance.
Spin–spin coupling: The interaction between neighbouring non-equivalent protons that causes the splitting of NMR signals into multiplets.
This interaction is transmitted through the σ-bonds connecting the atoms, generally across three bonds, meaning only protons on adjacent carbon atoms usually cause splitting.
The n + 1 Rule
The n + 1 rule stated in the OCR specification allows prediction of proton signal splitting based on the number of adjacent protons (n). The observed signal becomes a multiplet of n + 1 peaks.
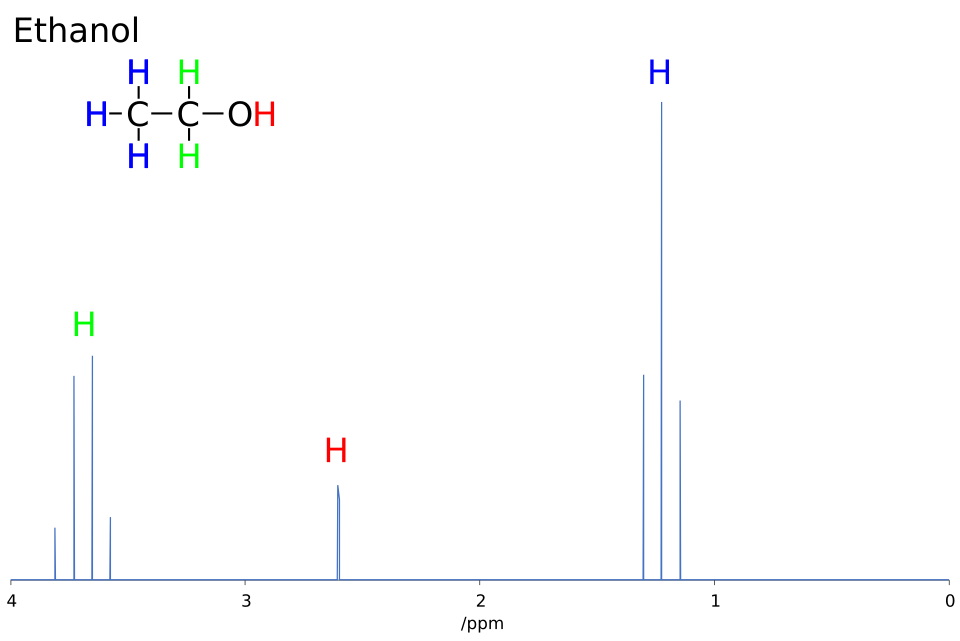
This ¹H NMR spectrum of ethanol shows how neighbouring protons split signals into multiplets. The CH₃ group appears as a triplet and the CH₂ group as a quartet, directly illustrating the n + 1 rule. Source
n + 1 rule: A rule stating that a proton with n neighbouring non-equivalent protons will split into a multiplet of n + 1 peaks.
In practice, this allows rapid structural deduction by linking peak patterns to likely bonding arrangements. After considering splitting, students should also evaluate relative peak areas and chemical shifts to confirm assignments.
Typical Multiplets and Their Structural Meaning
Splitting patterns follow predictable forms:
Singlet (1 peak)
No adjacent non-equivalent protons.
Doublet (2 peaks)
One neighbouring proton.
Triplet (3 peaks)
Two neighbouring protons.
Quartet (4 peaks)
Three neighbouring protons.
Multiplet (complex splitting)
Several neighbouring sets or asymmetrical coupling.
Coupling patterns for simple alkyl groups are particularly characteristic:
CH₃–CH₂– fragments often show a triplet for the CH₃ protons and a quartet for the CH₂ protons.
Isolated CH₃ groups bonded to quaternary carbons show singlets.
CH groups attached to different proton environments may appear as multiplets.
Coupling Constants (J-values)
Spin–spin interactions give rise to measurable separation between peaks called coupling constants. These are expressed in hertz (Hz) and reflect the strength of interaction between the coupled protons.
Coupling constant (J): The separation between peaks in a multiplet, measured in hertz, representing the strength of spin–spin coupling.
Coupling constants help distinguish overlapping patterns and confirm whether peaks arise from the same coupling system. For example, a doublet and triplet sharing the same J-value often come from neighbouring CH and CH₂ groups.
Aromatic systems show characteristic J-values that can indicate substitution patterns, but OCR A-Level primarily requires recognition of aromatic chemical-shift regions rather than advanced coupling analysis.
Aromatic Protons in Proton NMR
Aromatic rings create distinctive deshielded proton environments due to their circulating π-electron system, which induces an external magnetic field effect known as the ring-current effect. As a result, aromatic protons appear downfield relative to aliphatic protons.
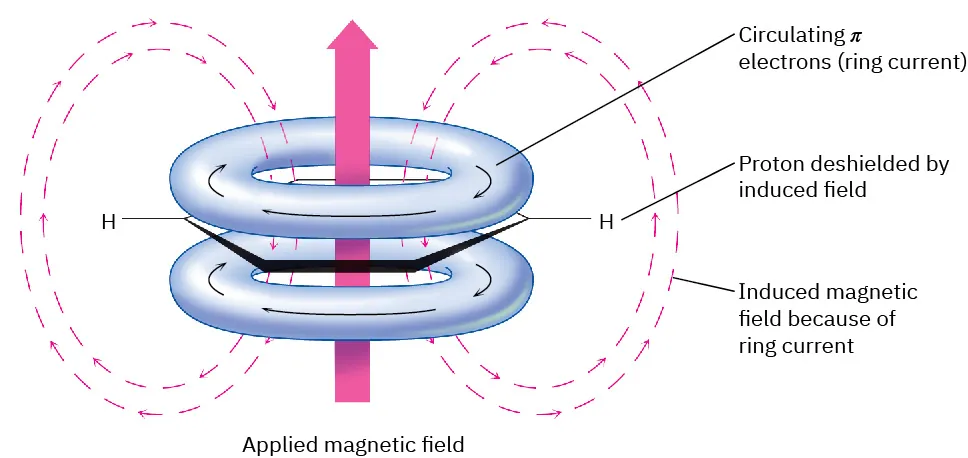
This diagram shows how circulating π electrons in an aromatic ring generate an induced magnetic field that deshields aromatic protons, shifting their signals downfield. Source
Aromatic Proton Chemical-Shift Range
According to the OCR specification, students must be able to recognise aromatic proton chemical shifts. Aromatic hydrogens typically resonate in a characteristic region:
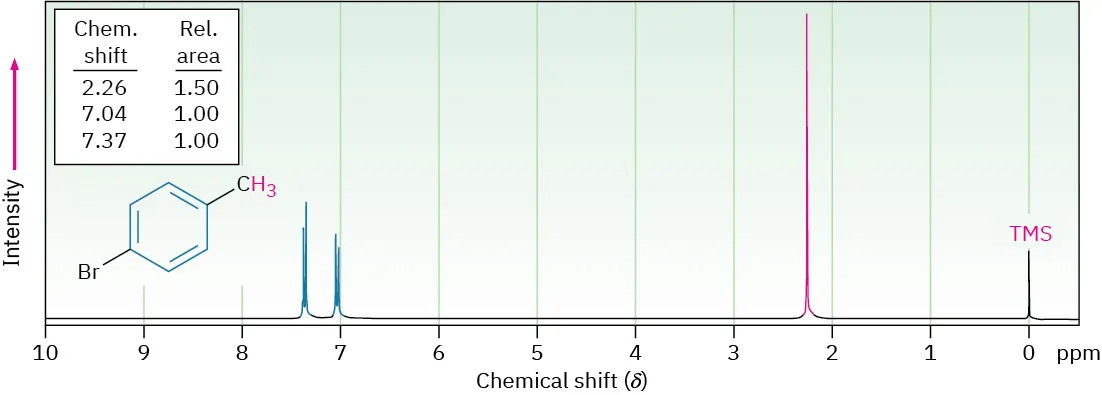
This ¹H NMR spectrum shows aromatic proton signals clustered around 7 ppm, a diagnostic feature of aromatic rings, alongside non-aromatic signals elsewhere in the spectrum. Source
Chemical shift range: δ 6.0–9.0 ppm
These values vary slightly depending on ring substituents:
Electron-withdrawing groups (e.g., NO₂, COOH) shift aromatic protons further downfield (closer to 8.5–9.0 ppm).
Electron-donating groups (e.g., OH, NH₂, alkyl groups) can shift some aromatic protons upfield (around 6.0–7.0 ppm).
Substitution pattern affects equivalence:
Ortho, meta, and para positions may produce distinct chemical shifts if they make the protons non-equivalent.
Why Aromatic Protons Are Deshielded
The ring-current effect plays a central role:
Ring-current effect: The magnetic effect generated by circulating π-electrons in an aromatic ring that causes aromatic protons to be deshielded and shifted downfield.
This phenomenon creates a predictable spectral signature, allowing rapid recognition of aromatic signals even when they form complex multiplets.
In-plane substituents and ring symmetry can also influence the degree of deshielding, but the fundamental requirement for OCR is the ability to identify aromatic resonances within their diagnostic ppm region.
Multi-Peak Patterns in Aromatic Regions
Although OCR does not require detailed aromatic coupling analysis, students should be aware that aromatic regions often contain overlapping multiplets due to the number of interacting protons around the ring. Aromatic protons can couple with several neighbours, often producing:
Multiplets with unclear symmetry
Clusters of peaks between 6–9 ppm
Characteristic downfield shifts marking aromatic presence
Key Points for Structural Identification Using Splitting and Aromatic Signals
When interpreting a proton NMR spectrum:
Check for splitting patterns using the n + 1 rule to determine the number of neighbouring protons.
Note J-values when available to confirm which peaks belong to the same coupling system.
Identify aromatic proton signals by their distinctive 6–9 ppm region.
Evaluate the number of aromatic protons to infer substitution patterns:
Five aromatic hydrogens often indicate a monosubstituted benzene ring.
Four or fewer hydrogens suggest di- or tri-substitution.
Combine splitting, chemical shift, and relative proton numbers to map out both aromatic and aliphatic structural features.
Understanding these principles enables construction of accurate structural proposals consistent with OCR exam expectations.
FAQ
Splitting only occurs between non-equivalent protons. If neighbouring protons are chemically equivalent, they do not split each other.
Protons may also fail to cause splitting if:
They are separated by more than three σ-bonds
Rapid proton exchange occurs (e.g. some O–H or N–H protons)
Molecular symmetry makes neighbouring protons equivalent
This explains why some signals appear as singlets despite nearby hydrogen atoms.
Aromatic protons usually have multiple neighbouring protons around the ring.
This leads to:
Coupling with more than one set of adjacent protons
Slightly different coupling constants for each interaction
Overlapping splitting patterns in the 6–9 ppm region
At A-Level, these signals are generally described as multiplets rather than analysed in detail.
In theory, aromatic protons follow spin–spin coupling rules, but in practice the n + 1 rule is difficult to apply.
This is because:
Aromatic protons often couple with more than two neighbours
Coupling constants vary around the ring
Signals overlap in a narrow chemical-shift range
For OCR A-Level, aromatic splitting patterns are not analysed using the n + 1 rule; recognising their chemical-shift region is sufficient.
Aromatic rings generate a strong ring-current effect when placed in a magnetic field.
This causes:
Increased deshielding of protons on the outside of the ring
A stronger induced magnetic field than in alkenes
Chemical shifts typically between 6–9 ppm
Alkenic protons experience less deshielding and usually appear between 4.5–6.5 ppm.
Splitting patterns in the aliphatic region reveal how many neighbouring protons are present.
For example:
A triplet and quartet pair indicates a CH₃–CH₂– group
A singlet may indicate a CH₃ group attached directly to the ring
Multiplets can suggest branching or multiple neighbouring environments
These patterns help identify the nature of substituents attached to the aromatic ring.
Practice Questions
A proton NMR spectrum contains a signal at δ 1.2 ppm that appears as a triplet.
a) State how many neighbouring protons are responsible for this splitting.
b) Name the rule used to predict this splitting pattern.
(2 marks)
a) Two neighbouring protons
1 mark
b) n + 1 rule
1 mark
A compound has the molecular formula C₈H₁₀O. Its proton NMR spectrum shows:
A multiplet between δ 7.1–7.4 ppm integrating to five protons
A quartet at δ 4.1 ppm integrating to two protons
A triplet at δ 1.3 ppm integrating to three protons
Use this information to explain what structural features are present in the molecule. Your answer should refer to splitting patterns and aromatic proton chemical shifts.
(5 marks)
Recognition that the multiplet at δ 7.1–7.4 ppm indicates aromatic protons
1 mark
Correct statement that five aromatic protons suggest a monosubstituted benzene ring
1 mark
Identification that the quartet at δ 4.1 ppm corresponds to protons with three neighbouring protons
1 mark
Identification that the triplet at δ 1.3 ppm corresponds to protons with two neighbouring protons
1 mark
Correct explanation that the quartet and triplet together indicate a CH₂–CH₃ group using the n + 1 rule
1 mark

