OCR Specification focus:
‘Deduce structures using elemental analysis, mass spectra, IR data, and NMR spectra together.’
Structural determination involves combining multiple analytical techniques. By integrating data from mass spectrometry, infrared absorption, elemental composition, and NMR spectroscopy, chemists reliably identify unknown organic molecules with high confidence.
Integrating Analytical Evidence for Structural Deductions
Determining an unknown organic structure requires building a consistent molecular picture from several complementary techniques. Each method provides distinct evidence about bonding, functional groups, atom connectivity, and molecular composition.
Elemental Analysis and Empirical Formula Determination
Elemental analysis provides the percentage composition of each element in a compound, enabling the empirical formula to be established.
Determining the Empirical Formula
Convert percentage composition into moles of each element.
Determine the simplest whole-number ratio.
Use this empirical formula as the foundation for subsequent structural evidence.
Normal sentence separating blocks.
Empirical Formula: The simplest whole-number ratio of atoms of each element present in a compound.
Elemental analysis narrows structural options but cannot provide information about functional groups or connectivity; these must come from spectroscopic evidence.
Mass Spectrometry: Establishing Molecular Mass and Fragmentation
Mass spectrometry provides the molecular ion peak (M⁺), which corresponds to the molecular mass of the entire molecule.
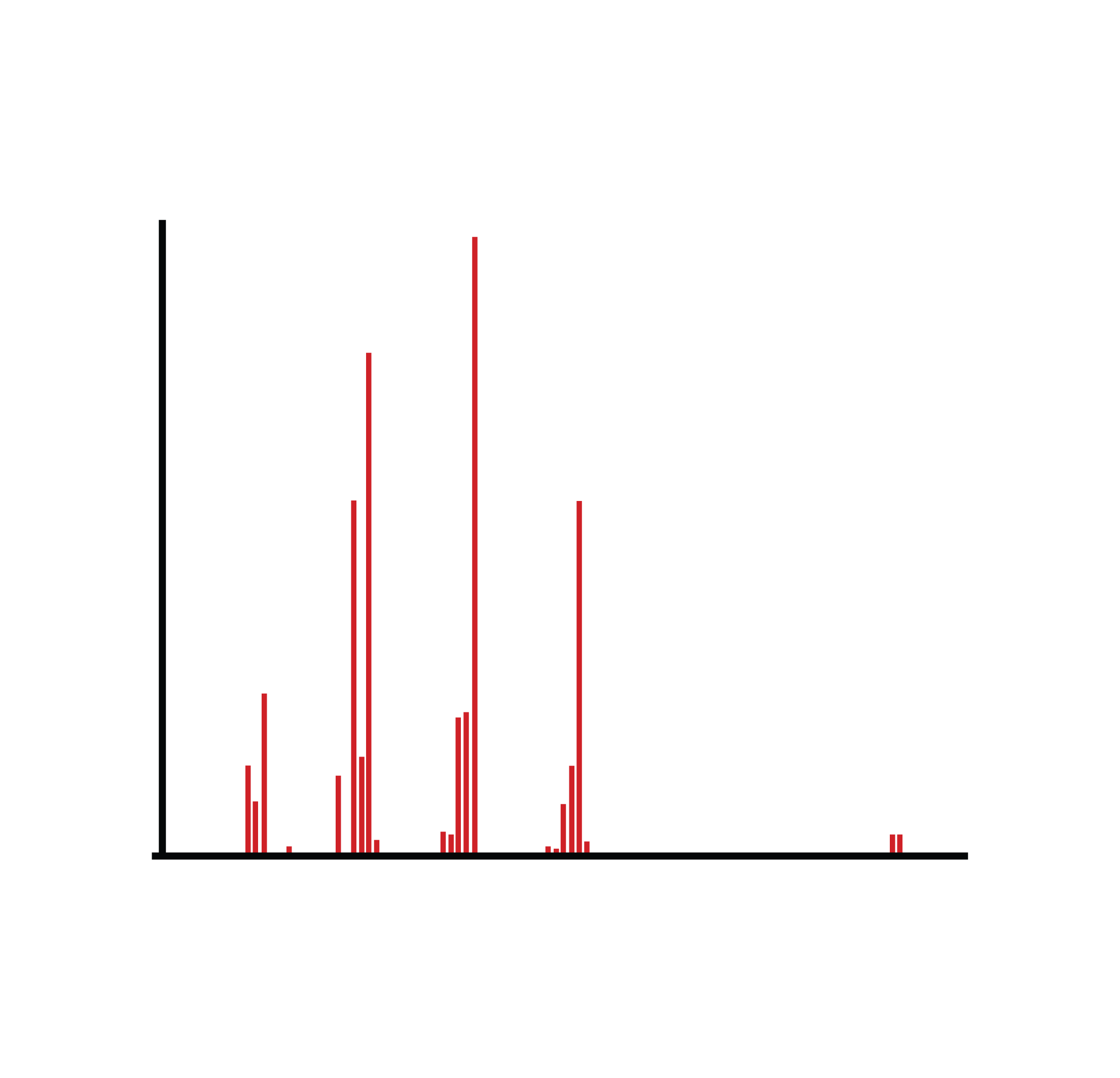
This diagram shows a typical mass spectrum plotting relative abundance against mass-to-charge ratio (m/z). The molecular ion peak and base peak provide essential information for determining molecular mass and assessing structural fragments. Source
Key Evidence from Mass Spectra
Molecular ion peak (M⁺): gives the molecular mass.
Fragmentation peaks: indicate the stability of potential fragments and suggest aspects of molecular structure.
Isotopic patterns: reveal the presence of halogens (e.g., Cl, Br).
Molecular Ion (M⁺): The ion formed when a molecule loses a single electron during mass spectrometry, giving its relative molecular mass.
A single sentence is placed here to maintain spacing before any further formatted block.
Fragmentation: The process in mass spectrometry where an ion breaks into smaller ions, producing characteristic peaks used to infer structural features.
Mass spectrometry suggests possible frameworks, but the functional groups must be identified using infrared spectroscopy.
Infrared (IR) Spectroscopy: Identifying Functional Groups
IR spectroscopy detects bond vibrations, providing strong evidence for functional groups.
Important IR Absorptions
Broad absorption around 3200–3600 cm⁻¹ → O–H
Sharp absorption around 1700 cm⁻¹ → C=O
Multiple peaks around 1600–1500 cm⁻¹ → aromatic ring
Using IR in Structural Deductions
Confirm or exclude key functional groups.
Distinguish between alcohols, carbonyls, carboxylic acids, amines, and alkenes.
Combine IR clues with mass spectral fragments to identify possible molecular motifs.
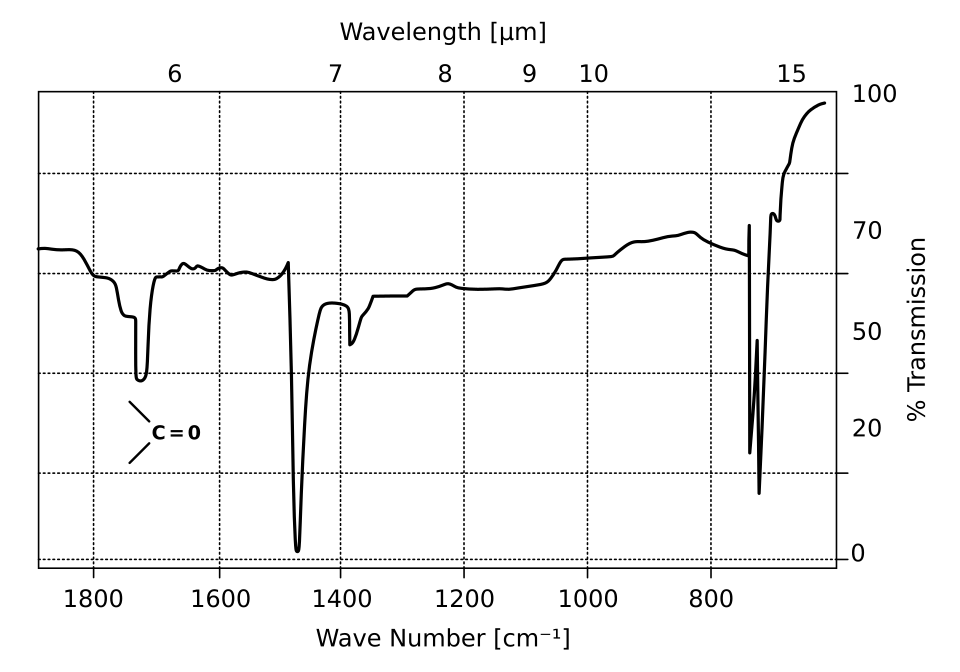
This IR spectrum highlights a strong carbonyl (C=O) absorption band used to confirm functional groups during structure deduction. The presence of this peak must be consistent with molecular mass and NMR evidence. Source
NMR Spectroscopy: Determining the Chemical Environment of Atoms
NMR spectroscopy is the most detailed tool for organic structural analysis, revealing chemical environments, connectivity, and relative numbers of atoms.
Carbon-13 (¹³C) NMR Evidence
Number of peaks = number of distinct carbon environments.
Chemical shift ranges indicate specific bond types (e.g., C=O near 200 ppm).
Chemical Environment: The electronic surroundings of a nucleus, influenced by neighbouring atoms and bonds, affecting its NMR chemical shift.
Proton (¹H) NMR Evidence
Proton NMR provides data on hydrogen environments and how they interact.
Key data obtained include:
Chemical shifts revealing proton types (e.g., aromatic, aldehydic, or alkyl).
Integration showing relative numbers of hydrogens.
Splitting patterns indicating the number of neighbouring protons via the n + 1 rule.
Essential Features for Structural Assembly
Singlets, doublets, triplets, multiplets reveal connectivity.
Aromatic proton shifts (around 6–9 ppm) confirm aromatic rings.
Exchangeable O–H or N–H protons may appear broad and may disappear upon D₂O addition.
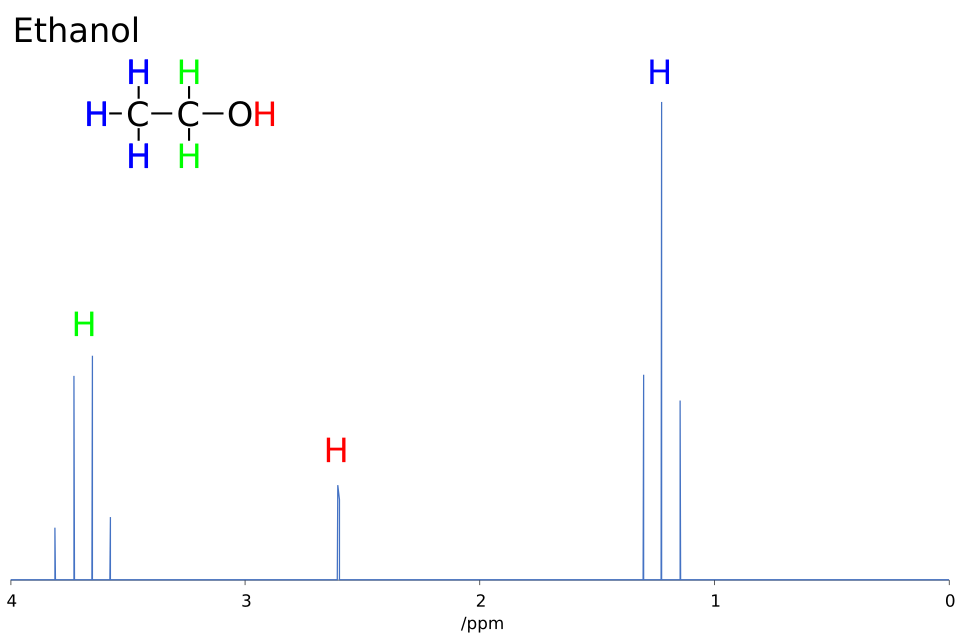
This diagram illustrates how neighbouring protons cause characteristic splitting patterns in ¹H NMR spectra. Splitting and integration data are essential for confirming hydrogen connectivity when deducing molecular structures. Source
Combining Spectroscopic Evidence Coherently
To deduce a full structure, evidence from every technique must support a single consistent molecular arrangement.
Step-by-Step Strategy for Structural Deduction
Use elemental analysis to determine empirical formula.
Use the M⁺ peak from mass spectrometry to find molecular mass and molecular formula.
Inspect fragmentation to narrow down possible carbon skeletons or functional groups.
Confirm functional groups using IR absorption peaks.
Use ¹³C NMR to infer the number and types of carbon environments.
Analyse ¹H NMR to determine hydrogen environments, integration patterns, and splitting rules.
Cross-check all information to eliminate inconsistent possibilities.
Common Structural Features Deduced from Combined Evidence
A strong C=O peak in IR combined with a carbonyl shift in ¹³C NMR indicates a carbonyl-containing functional group.
Aromatic proton signals in ¹H NMR support aromatic IR absorptions and mass-spectral patterns.
Fragmentation peaks (e.g., loss of 15 for CH₃) help identify substituent groups.
Building a Consistent Molecular Proposal
A complete structural determination requires convergence of all data. A viable structure must satisfy:
The empirical and molecular formulae, including degrees of unsaturation.
Functional groups confirmed by IR absorptions.
Bonding and carbon environments indicated by ¹³C NMR.
Proton environment details, including splitting and integration, shown in ¹H NMR.
Fragmentation pathways consistent with mass-spectral peaks.
Through systematic evaluation and cross-referencing, an unambiguous organic structure can be proposed using elemental analysis, mass spectra, IR data, and NMR spectra together.
FAQ
Each analytical technique describes a different aspect of the same molecule. If any piece of data contradicts the molecular formula, the proposed structure must be incorrect.
For example, a structure containing a carbonyl group cannot be correct if the molecular formula lacks sufficient oxygen atoms. Consistency across all evidence is essential.
Degrees of unsaturation indicate the total number of rings and multiple bonds present in a molecule.
This helps to:
Eliminate structures with too many or too few double bonds
Predict whether rings or carbon–carbon double bonds must be present
Check consistency with IR and NMR data
It is particularly useful when multiple isomers share the same molecular formula.
NMR provides the most detailed information about atom connectivity and chemical environments.
Mass spectrometry and IR narrow down molecular mass and functional groups, while NMR confirms how atoms are arranged. This makes NMR ideal for final structural confirmation rather than initial screening.
Frequent errors include:
Ignoring contradictory data from one technique
Over-relying on a single spectrum, usually NMR
Forgetting to check integration ratios against the molecular formula
Proposing structures that fit some evidence but not all
Careful cross-checking prevents these mistakes.
Structural isomers share the same molecular formula but differ in atom arrangement.
Mass spectrometry confirms molecular mass, IR identifies functional groups, and NMR reveals connectivity. Only one isomer will fit all these data sets simultaneously, allowing a single correct structure to be deduced.
Practice Questions
An unknown organic compound has the molecular formula C3H6O. Its IR spectrum shows a strong absorption at approximately 1700 cm⁻¹.
(a) Identify the functional group present.
(b) State one piece of additional spectroscopic evidence that would help distinguish between two possible isomers of this compound.
(2 marks)
(a)
Correct identification of a carbonyl (C=O) functional group (1 mark)
(b)
Any one correct piece of evidence, such as:
Use of ¹H NMR to compare splitting patterns or integration
Use of ¹³C NMR to compare the number of carbon environments
(1 mark)
An organic compound contains carbon, hydrogen, and oxygen only.
Elemental analysis gives an empirical formula of C2H4O.
The mass spectrum shows a molecular ion peak at m/z = 88.
The IR spectrum shows a broad absorption around 3200–3600 cm⁻¹ but no absorption near 1700 cm⁻¹.
The ¹H NMR spectrum shows two signals with an integration ratio of 3:1.
Use all the information provided to deduce a possible structure for the compound. You should refer to each analytical technique in your answer.
(5 marks)
Correct determination of molecular formula C4H8O2 from empirical formula and molecular ion peak (1 mark)
Correct interpretation of IR data indicating presence of an O–H group and absence of a carbonyl (1 mark)
Correct use of ¹H NMR integration (3:1) to identify two proton environments with relative numbers (1 mark)
Logical combination of all data to propose a structure consistent with an alcohol or diol (1 mark)
Clear reference to how at least three techniques together support the proposed structure (1 mark)

