OCR Specification focus:
‘Explain TMS as the chemical-shift standard, deuterated solvents, and D₂O exchange for O–H/N–H.’
Introduction (25 words) NMR spectroscopy relies on consistent reference signals and appropriate solvents. Understanding standards, solvent choices, and exchange processes ensures accurate interpretation of proton and carbon-13 spectra.
NMR Standards and Solvents
Nuclear Magnetic Resonance (NMR) spectroscopy requires stable reference points and chemically appropriate solvents to generate interpretable spectra. OCR expects students to understand why tetramethylsilane (TMS) is used as the chemical-shift standard, why deuterated solvents are essential for solution-state NMR, and how D₂O exchange provides crucial evidence for identifying O–H and N–H groups. These concepts support analysis across proton and carbon-13 spectra.
Tetramethylsilane (TMS) as the Chemical-Shift Standard
TMS is universally used as the reference compound for chemical shift measurements. When introduced for the first time, the term must be defined clearly.
Tetramethylsilane (TMS): A reference compound producing a single, sharp NMR peak at 0 ppm, used to calibrate chemical shifts in NMR spectroscopy.
TMS provides a 0 ppm standard because:
All twelve protons in TMS are chemically identical, giving one intense, sharp signal.
TMS is chemically inert, so it does not react with typical organic samples.
TMS is volatile, making it easy to remove after measurements.
Its protons are highly shielded, ensuring a peak at the extreme right of the spectrum, away from most sample signals.
These qualities allow the instrument to set the TMS signal as zero, enabling all other chemical shifts to be measured relative to a consistent baseline.
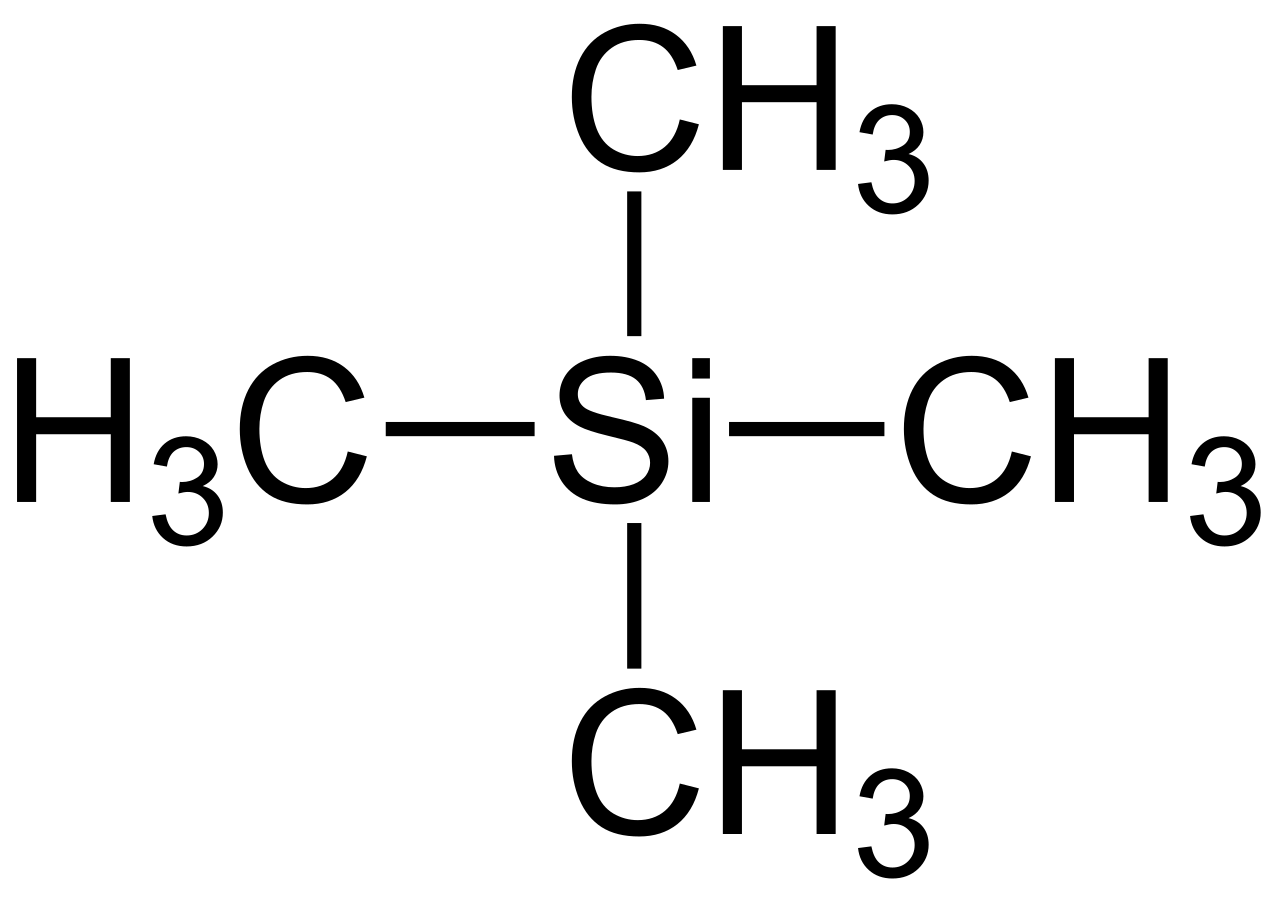
Structural formula of tetramethylsilane (TMS), the universal 0 ppm reference in NMR. Its protons are highly shielded, so the signal appears upfield compared with most organic compounds. Source
Deuterated Solvents
A major issue in proton NMR is that ordinary solvents contain hydrogen, which would produce large, interfering peaks. Deuterated solvents resolve this problem.
Deuterated solvent: A solvent in which hydrogen atoms are replaced with deuterium atoms (²H or D), preventing interference in proton NMR spectra.
A normal sentence is required here. Deuterium resonates at a completely different frequency from ¹H, so its signals do not appear in standard proton NMR spectra.
Common deuterated solvents include:
CDCl₃ (deuterated chloroform) — the most widely used
D₂O (deuterium oxide)
CD₃OD (deuterated methanol)
C₆D₆ (deuterated benzene)
Why deuterated solvents are essential
Deuterated solvents serve several critical functions:
Preventing solvent signal interference
Regular solvents produce large proton signals, overshadowing analyte peaks.
Deuterated solvents contain D instead of H, eliminating this issue.
Allowing field-frequency lock
NMR spectrometers use the deuterium signal to maintain a stable magnetic field during data collection.
Offering chemical compatibility
Many organic samples dissolve well in common deuterated solvents.
Residual solvent peaks
Even deuterated solvents contain tiny amounts of undeuterated molecules (e.g., CHCl₃ in CDCl₃).
These produce small residual solvent peaks that are well documented and easily recognised.
Students must learn their typical positions, such as CDCl₃ showing a residual peak around 7.26 ppm in proton NMR.

A labelled bottle of deuterated chloroform (CDCl₃), one of the most common NMR solvents. Deuteration reduces solvent ¹H signals and provides a deuterium signal used for the field-frequency lock. Source
D₂O Exchange for O–H and N–H Identification
The OCR specification emphasises the utility of D₂O exchange for confirming exchangeable protons. Functional groups like alcohols, carboxylic acids, and amines contain protons attached to electronegative atoms, making them labile.
D₂O exchange: A process in which exchangeable O–H or N–H protons are replaced by deuterium when D₂O is added, causing their ¹H NMR signals to disappear.
A normal sentence must follow. This technique provides strong evidence for identifying groups containing heteroatom-bound hydrogen.
How D₂O exchange works
When D₂O is added to an NMR sample:
The sample’s O–H or N–H protons exchange with D from D₂O.
The original proton resonance disappears from the ¹H NMR spectrum.
The newly formed O–D or N–D bond no longer produces a detectable proton signal.
Applications in structural identification
Alcohols
O–H peaks often appear broad and variable.
If the peak disappears after D₂O addition, the proton was exchangeable.
Carboxylic acids
The acidic O–H proton shows a very broad signal.
Loss of this peak confirms the functional group.
Primary and secondary amines
N–H protons can appear as one or two signals depending on substitution.
Disappearance following D₂O exchange distinguishes amines from non-amine environments.
Important features of exchangeable protons
They do not follow the n + 1 splitting rule reliably because they usually exchange too rapidly.
Their chemical shifts vary widely depending on hydrogen bonding, solvent, and concentration.
D₂O exchange offers a reliable method of confirmation independent of peak shape or position.
Limitations and considerations
The sample must be compatible with trace water present in D₂O.
Strongly basic or acidic samples may cause partial decomposition of the solvent or analyte.
D₂O exchange is mainly used in proton NMR and is not typically relevant for carbon-13 spectra.
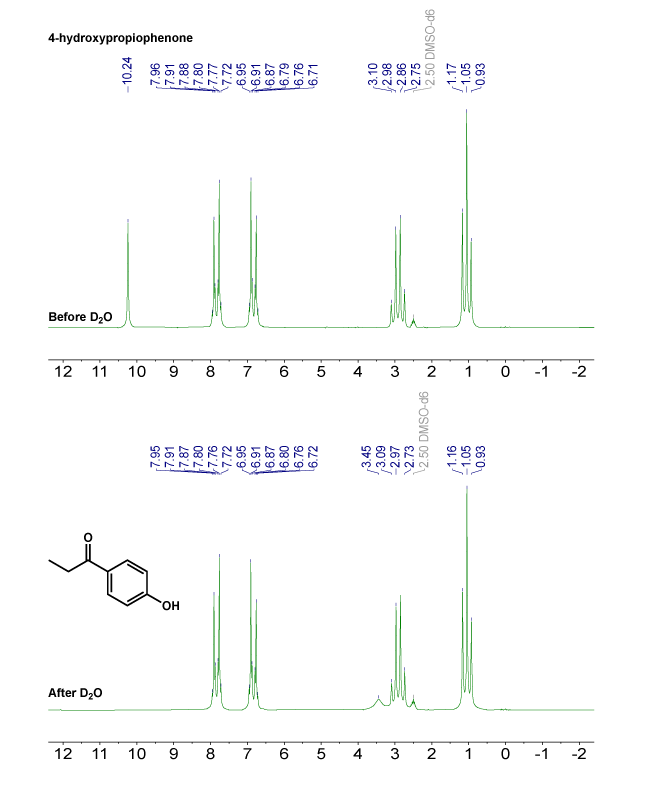
¹H NMR spectra before and after adding D₂O show the diagnostic disappearance of an exchangeable –OH signal. This confirms which resonance belongs to an –OH (or similarly, –NH) proton; the water/HOD signal shown is beyond the minimum syllabus requirement. Source
Summary of Key Points Covered through Specification
This section of the OCR specification requires understanding of:
TMS as a chemical-shift standard and why it defines 0 ppm.
Deuterated solvents and their necessity in eliminating proton interference.
D₂O exchange as essential evidence for identifying O–H and N–H functional groups in ¹H NMR spectra.
These foundational elements enable correct interpretation of NMR data and support structure determination across organic chemistry topics.
FAQ
Chemical shift values are measured relative to an agreed reference point rather than an absolute scale.
TMS is assigned 0 ppm by convention because its protons are more shielded than almost all organic protons. This places its signal at the extreme upfield end of the spectrum.
Using 0 ppm avoids negative chemical shifts and ensures consistency across laboratories, instruments, and publications worldwide.
O–H and N–H protons undergo rapid exchange between molecules, especially in the presence of trace water.
This exchange disrupts consistent spin–spin coupling, leading to broad, poorly defined peaks rather than sharp multiplets.
Hydrogen bonding further increases variability in chemical environment, contributing to peak broadening and inconsistent splitting patterns.
D₂O can act both as a solvent and as an exchange reagent.
When used as the solvent, all exchangeable protons are replaced by deuterium before the spectrum is recorded, so O–H and N–H peaks are absent from the start.
This is useful when the focus is on carbon-bound protons only, but it removes diagnostic information that could confirm functional groups.
Deuterated solvents are never 100% deuterated.
Small amounts of undeuterated molecules remain, producing weak but detectable proton signals known as residual solvent peaks.
These peaks appear at known chemical shifts and are predictable, allowing chemists to recognise and ignore them during spectral interpretation.
C–H bonds are much stronger and less polar than O–H or N–H bonds.
As a result, hydrogen atoms bonded to carbon do not exchange with deuterium under normal NMR conditions.
This selectivity makes D₂O exchange a powerful diagnostic tool, as only heteroatom-bound hydrogens are affected, leaving the rest of the spectrum unchanged.
Practice Questions
Tetramethylsilane (TMS) is added to an organic compound before recording its proton NMR spectrum.
Explain why TMS is used as the chemical-shift standard.
(2 marks)
Award one mark for each correct point.
TMS produces a single sharp signal because all protons are in the same environment.
The TMS signal is assigned a chemical shift of 0 ppm, providing a reference for all other peaks.
Accept: protons in TMS are highly shielded so the peak appears at the extreme upfield end of the spectrum.
(Any two points for full marks.)
A student records the proton NMR spectrum of an organic compound using a deuterated solvent. A drop of D₂O is then added and the spectrum is recorded again.
(a) Explain why a deuterated solvent is used when recording proton NMR spectra.
(b) Describe what happens to O–H or N–H signals after the addition of D₂O.
(c) Explain how the observation in part (b) helps identify functional groups in the compound.
(5 marks)
(a) Deuterated solvent (2 marks)
Prevents the solvent producing large proton NMR signals that would interfere with the spectrum.
Deuterium does not give a signal in proton NMR / allows the spectrometer to lock using deuterium.
(b) Effect of D₂O addition (2 marks)
O–H or N–H proton signals disappear or significantly reduce in the proton NMR spectrum.
Due to exchange of hydrogen with deuterium.
(c) Identification of functional groups (1 mark)
Disappearance of a peak confirms the presence of an exchangeable proton, identifying an –OH or –NH functional group.
(Maximum 5 marks.)

