OCR Specification focus:
'Specification information for this subsubtopic: Safely and correctly use a range of practical equipment and materials, identifying hazards and understanding how to minimise risks.'
Introduction
Chemistry practical work demands precision and safety. Understanding how to safely and correctly use laboratory equipment and materials ensures reliable results and protects all individuals in the laboratory environment.
Safe Use of Equipment in the Laboratory
General Laboratory Safety Principles
Before conducting any experiment, it is essential to recognise the potential hazards associated with the materials and apparatus in use. This includes understanding chemical, physical, and procedural risks.
Key principles include:
Read all experimental instructions thoroughly before beginning.
Wear appropriate PPE (personal protective equipment), such as lab coats, goggles, and gloves.
Keep benches clear of unnecessary materials to reduce risk of spills or contamination.
Ensure familiarity with emergency equipment, including eyewash stations, fire extinguishers, and safety showers.
Hazard: A potential source of harm or adverse health effect on a person.
Risk: The likelihood that harm will occur from exposure to a hazard.
Every experiment involves identifying hazards and assessing associated risks. For example, heating organic solvents introduces both flammability and vapour inhalation risks, requiring appropriate precautions like working in a fume cupboard.
Correct Handling of Chemicals
Chemical Labelling and Classification
Chemicals must always be correctly labelled according to the Globally Harmonised System (GHS), which uses standard hazard pictograms and signal words such as Danger or Warning.
When handling chemicals:
Read the Safety Data Sheet (SDS) for each reagent before use.
Never mix or decant chemicals without understanding possible reactivity hazards.
Clearly label all containers, especially diluted solutions and waste bottles.
Use secondary containment trays when handling corrosive or toxic substances.
Identify GHS hazard pictograms on labels and consult the SDS before use.

Official GHS “Corrosion” pictogram indicating substances that corrode metals and cause severe skin/eye burns. Recognising this symbol supports correct PPE selection, careful dispensing, and fume-hood use for volatile or irritating fumes. Source
Safety Data Sheet (SDS): A document providing information on a chemical’s hazards, handling, storage, and emergency measures.
Common chemical hazard types include:
Corrosive (e.g. concentrated acids, alkalis) – cause burns to skin or materials.
Irritant (e.g. dilute ammonia) – cause mild inflammation.
Toxic (e.g. lead salts) – cause serious health effects upon exposure.
Flammable (e.g. ethanol) – ignite easily near a heat source.
Oxidising (e.g. potassium manganate(VII)) – intensify combustion.
Minimising Risks in Practical Work
Risk Assessment Process
A structured risk assessment identifies hazards, evaluates risk levels, and determines suitable control measures.
Typical stages include:
Identify hazards (chemicals, equipment, procedures).
Assess who might be harmed and how.
Evaluate the level of risk (low, medium, high).
Implement control measures (e.g. fume hood use, PPE).
Record findings and review before each practical.
Control measures can include:
Engineering controls: fume cupboards, safety screens, heat-resistant mats.
Administrative controls: standard operating procedures, supervision, training.
Personal controls: gloves, goggles, appropriate clothing.
Safe Use of Common Laboratory Equipment
Glassware
Glassware such as beakers, pipettes, burettes, and flasks should always be checked for cracks or chips before use. Damaged glass may shatter when heated or under pressure.
Always clamp glassware securely when heating or assembling apparatus.
When inserting glass tubing into bungs, lubricate the tubing and use protective gloves.
Allow hot glass to cool before touching or washing to avoid burns or breakage.
Heating Apparatus
Safe heating requires understanding the heat source and material properties.
Bunsen burners should always be lit with the air hole closed and on a heatproof mat.
Use tongs or heatproof gloves when handling hot apparatus.
Never heat a closed container — this can cause pressure buildup and explosion.
Alternatives such as electric heaters or water baths can reduce fire risks.
Handling Equipment for Measurement and Analysis
Balances and Measuring Cylinders
When recording mass, ensure the balance is on a stable surface and free from vibration. Avoid spilling chemicals on the pan and always tare the balance before measuring.
When measuring volumes, read the meniscus at eye level and use graduated equipment appropriate to the precision required (e.g. burettes for titrations).
Read volumes at eye level, using the bottom of the meniscus for colourless liquids.
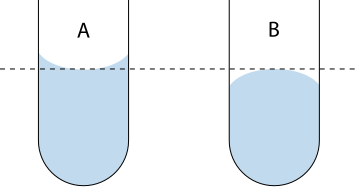
Labeled diagram showing correct eye-level alignment and the meniscus in a cylinder. It illustrates why readings taken above or below eye level are inaccurate. Vector artwork scales cleanly for print or web without loss of quality. Source
Meniscus: The curved surface of a liquid in a container; measurements should be taken at the lowest point of the curve.
Avoid cross-contamination by rinsing pipettes and burettes with the solution to be used, not with water, before filling.
Safe Handling and Storage of Materials
Storage Procedures
Chemicals must be stored according to their compatibility and hazard class. For instance:
Acids and bases stored separately to prevent neutralisation reactions.
Flammable liquids kept in ventilated, fire-resistant cabinets.
Oxidising agents isolated from organic materials and fuels.
Always ensure containers are clearly labelled, sealed tightly, and dated upon opening. Outdated reagents should be disposed of following local waste protocols.
Disposal of Waste Materials
Improper disposal can cause contamination and injury.
Aqueous waste: neutralise acids and bases before disposal.
Organic waste: place in designated solvent waste containers.
Solid waste: label and seal before sending to chemical disposal.
Glass waste: use a dedicated sharps bin.
Special Precautions for High-Risk Materials
Corrosive and Toxic Substances
Handle concentrated acids and alkalis with gloves and eye protection. Always add acid to water, not vice versa, to prevent exothermic splashes.
Toxic materials such as heavy metal salts or organic solvents should be handled under a fume hood to prevent inhalation.
Work at least 15 cm inside the fume hood and keep the sash at the indicated safe height.
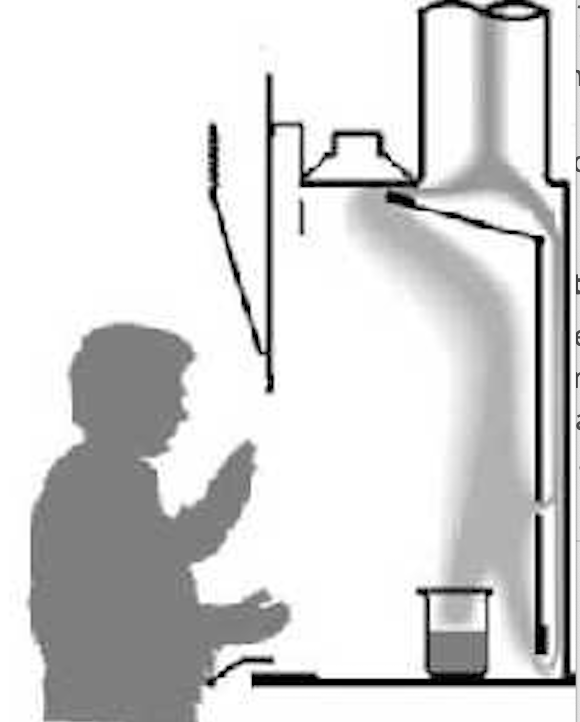
Illustration of correct equipment placement to optimise containment in a chemical fume hood. Positioning apparatus well behind the sash plane reduces eddies and leakage at the hood face. The source page also shows poor and good placements for comparison. Source
Flammable and Volatile Substances
Keep away from open flames and spark sources.
Use minimal quantities during experiments.
Replace lids immediately after use to reduce vapour exposure.
Dispose of contaminated materials in metal bins with tight-fitting lids.
Good Laboratory Practice (GLP)
Good Laboratory Practice ensures reliability and reproducibility in experimental results through consistent procedures and documentation.
Calibrate equipment regularly (balances, thermometers, pH meters).
Maintain accurate records of reagent batches, equipment checks, and waste disposal.
Ensure all users are trained in emergency response and first aid protocols.
By adhering to these practices, chemists demonstrate both competence and responsibility in experimental science — key outcomes of this OCR A-Level Chemistry skill area.
FAQ
Immediately alert others and your teacher, then contain the spill only if it is safe to do so. For liquid chemicals, use spill kits or absorbent materials to prevent spreading.
Avoid direct contact with the substance and never attempt to neutralise an unknown chemical.
If the spill involves corrosive or toxic materials, evacuate the area and follow the school’s emergency protocol.
Always wash affected skin thoroughly and report the incident promptly.
Cracked or chipped glassware can shatter when exposed to heat or sudden temperature changes. This poses risks of injury and contamination.
Before use:
Inspect glassware for cracks, scratches, or star-shaped fractures.
Discard any damaged equipment immediately.
Use borosilicate glass (e.g. Pyrex) for heating as it expands evenly under temperature changes.
Regular inspection reduces accidents and ensures accurate experimental results.
Cross-contamination alters concentrations and produces inaccurate results. To prevent it:
Rinse pipettes and burettes with the solution to be used, not with water.
Use clean spatulas or separate droppers for each reagent.
Label all beakers and flasks clearly.
Ensure all measuring instruments are washed and dried between uses, and never return unused reagents to stock bottles.
A fume hood protects the user from hazardous vapours by drawing air away and venting it outside. It is used when working with toxic, flammable, or volatile chemicals.
A laminar flow cabinet, by contrast, protects the sample rather than the user. It directs filtered air over the work surface to prevent contamination in microbiological or analytical work.
Never substitute one for the other without confirming their intended safety function.
Calibration ensures that equipment such as balances, thermometers, and pH meters provides accurate readings.
Inaccurate instruments can lead to systematic errors and unreliable results. Calibration typically involves comparing readings with known standards and adjusting instruments accordingly.
Regular calibration is part of Good Laboratory Practice (GLP) and helps maintain consistency across experiments, especially when results are compared or repeated.
Practice Questions
State two safety precautions you should take when handling concentrated sulfuric acid during a laboratory experiment. (2 marks)
1 mark for wearing appropriate personal protective equipment (e.g. gloves, goggles, lab coat).
1 mark for adding acid to water rather than water to acid when diluting, to prevent exothermic splashes.
A student is instructed to prepare and heat a solution containing ethanol in a school laboratory. Describe the safety measures that should be taken when carrying out this procedure. In your answer, refer to the correct use of equipment and materials. (5 marks)
1 mark for identifying ethanol as flammable and stating that it must be kept away from open flames/Bunsen burners.
1 mark for suggesting the use of a water bath or electric heater instead of direct flame heating.
1 mark for wearing appropriate PPE such as safety goggles and lab coat to prevent chemical contact.
1 mark for conducting the procedure in a well-ventilated area or fume hood to avoid inhalation of vapours.
1 mark for ensuring all containers are clearly labelled and securely closed to minimise evaporation and contamination.

