OCR Specification focus:
‘Follow written instructions; make and record observations and measurements accurately; keep appropriate records of experimental activities.’
Introduction
Accurate recording and careful following of instructions are vital in A-Level Chemistry practical work. These skills ensure results are reliable, reproducible, and scientifically valid, forming the foundation of experimental chemistry.
Following Written Instructions
Importance of Following Instructions
In chemistry, precision and consistency are essential. Experiments often involve complex procedures, where misunderstanding a step or misreading an instruction can lead to inaccurate data, failed reactions, or safety risks.
Students must:
Read experimental procedures fully before starting, identifying materials, reagents, and apparatus needed.
Highlight key steps such as heating, measuring, or timing that are crucial to data accuracy.
Understand the purpose of each step — this supports problem solving if an unexpected result occurs.
Interpreting Experimental Procedures
Written instructions in laboratory contexts may use technical terminology. Students should recognise command words such as:
Measure: implies quantitative recording with suitable precision.
Observe: denotes qualitative recording (e.g. colour change, gas evolution).
Record: means to document results clearly and systematically.
Accuracy: The closeness of a measured value to the true or accepted value.
A high level of accuracy is dependent on correct interpretation and execution of procedural details.
Practical Independence
The OCR specification requires students to demonstrate independent working during experiments. This means being able to:
Identify potential errors or inconsistencies during an experiment.
Adjust apparatus setup (e.g. retighten a clamp stand or correct meniscus reading).
Decide when data collection should be repeated for consistency.
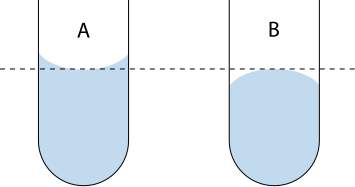
A labelled diagram showing how to read a concave meniscus at eye level to avoid parallax error. The correct sightline intersects the lowest point of the meniscus, ensuring precision in volume measurement. Source
Independence reflects genuine understanding, not rote following of steps.
Making Observations
Qualitative Observations
Observations involve detecting and describing visible or sensory changes. In chemistry, these may include:
Colour changes (e.g. blue to green for copper(II) reactions).
Formation of precipitates.
Gas evolution (bubbling or effervescence).
Temperature changes (endothermic or exothermic).
Observation: The systematic noting and recording of phenomena as they occur in an experiment.
Accurate observation requires consistency in descriptive terms — for example, writing “pale blue precipitate formed” rather than “blue solid appeared.”
Quantitative Observations
Quantitative data involves numerical measurement of chemical quantities. Students must select appropriate measuring devices depending on required precision, such as:
Burette or pipette for titration volumes (±0.05 cm³ precision).
Thermometer or digital probe for temperature (±0.1 °C precision).
Analytical balance for mass (±0.001 g precision).
Recording Measurements
General Principles
Recording data properly is essential to maintain data integrity and allow verification by others. All entries should be made in ink within a laboratory notebook or results table. Erasures should be avoided — instead, draw a single line through errors and correct beside them.

A real laboratory notebook entry demonstrating clear titles, dated sections, methods detailed enough for replication, and raw data. This visual reinforces best practice for permanent, transparent records. Source
Essential components include:
Clear headings and units (e.g. “Temperature / °C”, “Volume / cm³”).
Consistent decimal places to reflect measuring precision.
Immediate recording of results to prevent data loss.
Measurement Error: The difference between a measured value and the true value of the quantity being measured.
Students should identify possible sources of systematic and random errors during recording.
Types of Data
There are two main data types relevant to chemical experiments:
Raw data: Direct measurements taken during the experiment (e.g. titration volumes).
Processed data: Calculated values derived from raw data (e.g. mean titre, rate constants).
All raw data should remain unaltered to allow later verification.
Using Appropriate Units
Units must conform to SI conventions, ensuring comparability between experiments.
Common units include:
Mass: grams (g) or kilograms (kg)
Volume: cubic centimetres (cm³) or litres (dm³)
Temperature: degrees Celsius (°C) or Kelvin (K)
Time: seconds (s)
Keeping Appropriate Experimental Records
Maintaining Laboratory Records
Laboratory records form a permanent record of experimental activity. A well-maintained record includes:
Title and date of experiment.
Aim or purpose.
Apparatus list and chemical reagents with quantities.
Method, including procedural notes and any deviations.
Recorded data (tables and observations).
Data analysis and interpretation (brief).
Students should never rewrite results after the experiment; all real-time notes must remain visible to preserve authenticity.
Data Integrity and Reliability
Data integrity refers to the accuracy, completeness, and consistency of recorded information. To ensure reliability:
Repeat experiments to confirm reproducibility.
Cross-check results with peers or reference values.
Note all anomalies and potential causes.
Reproducibility: The ability to obtain consistent results when an experiment is repeated under similar conditions by different people.
Ethical and Safety Considerations
Following instructions and recording data also includes ethical responsibility. Students must not fabricate or selectively report data. All modifications to procedure or unexpected results should be recorded honestly.
Safety must be maintained by:
Complying with provided risk assessments and COSHH regulations.
Wearing correct personal protective equipment (PPE).
Ensuring accurate labelling of chemicals and samples.
Digital and Graphical Recording
Using Data Tables and Graphs
Accurate tabulation helps identify patterns and anomalies quickly.
Tables should:
Use clear column headings with units.
Align decimal places correctly.
Include means or averages only where appropriate (e.g. titration results excluding anomalous titres).
Graphs should:
Be plotted using appropriate scales.
Label axes with units.
Draw best-fit lines for continuous data and point-to-point lines for discontinuous data.
Gradient (m) = Δy / Δx
Δy = Change in dependent variable (with unit)
Δx = Change in independent variable (with unit)
Gradients are crucial in determining reaction rates or temperature coefficients, so accurate data recording directly affects subsequent analysis.
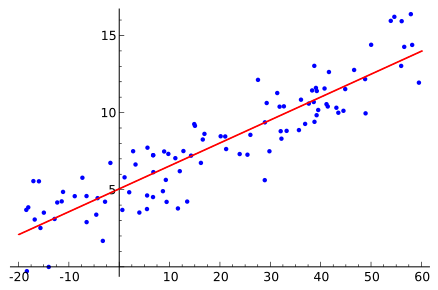
A simple scatter plot with a fitted straight line illustrating clear axes, data markers, and a regression line that communicates trend without clutter. This visual reinforces accurate presentation of numerical data. Source
Digital Data Tools
Modern laboratories often use data loggers, spreadsheets, and graphing software. These tools:
Reduce human error in reading instruments.
Enable quick processing of large datasets.
Facilitate data storage and retrieval for moderation or review.
However, students should still understand manual recording techniques, as electronic devices can fail or introduce systematic biases if not calibrated correctly.
Final Emphasis
Following instructions and recording data accurately form the backbone of reliable chemistry practical work. Mastery of these skills demonstrates competence, independence, and scientific integrity — aligning directly with OCR’s expectations for practical endorsement.
FAQ
If an instruction seems unclear, consult your teacher or laboratory supervisor before proceeding. Misinterpreting a step could affect both your safety and the accuracy of your data.
You can also:
Re-read the context of the instruction within the experiment.
Compare it with other reliable sources, such as the OCR practical handbook.
Record in your notes any clarifications received to maintain transparency.
External factors such as temperature, humidity, and light intensity can influence chemical reactions and measurements. Recording these ensures reproducibility and allows other scientists to interpret or replicate your results accurately.
For example, changes in temperature can alter gas volume readings or affect reaction rates, so environmental details help explain any anomalies.
Never erase or cover up errors. Instead, draw a single line through the incorrect data so it remains visible. Then write the correction clearly beside it with a short note explaining why it was changed.
This maintains integrity in your records and ensures the audit trail of all experimental data remains intact.
Qualitative data should use precise, objective language that avoids personal interpretation.
Examples of best practice:
Use clear descriptors such as “pale yellow solution turned colourless” rather than “looked different.”
Record the time or stage in the experiment when the observation occurred.
Write observations immediately to avoid memory errors.
Using consistent apparatus ensures that data is comparable and that any systematic error remains constant across all trials.
If different measuring devices are used, small variations in calibration or precision could distort overall results.
Maintaining consistency in equipment type, calibration, and handling method increases data reliability and supports fair experimental comparison.
Practice Questions
A student is instructed to measure 25.0 cm³ of hydrochloric acid using a pipette. Explain how the student should ensure the measurement is both accurate and reliable. (2 marks)
1 mark for describing reading the meniscus at eye level to avoid parallax error.
1 mark for mentioning rinsing the pipette with the solution before use or using consistent technique to ensure reliability.
During a titration experiment, a student is asked to record all results in their laboratory notebook. Explain the importance of following written instructions accurately and describe how results should be recorded to meet OCR practical requirements. (5 marks)
1 mark for stating that following instructions ensures reliable and reproducible results.
1 mark for noting that all observations and measurements must be recorded immediately in ink.
1 mark for explaining that entries should include units and consistent decimal places.
1 mark for stating that no results should be erased or rewritten; corrections should be crossed through once and rewritten clearly.
1 mark for mentioning that maintaining clear and complete records demonstrates scientific integrity and allows verification by others.

