OCR Specification focus:
‘Use a wide range of experimental instruments, equipment and techniques appropriate to the specification’s knowledge and understanding.’
In OCR A-Level Chemistry, mastering diverse laboratory instruments and techniques develops precision, accuracy, and confidence, allowing students to collect valid data and demonstrate high-level experimental competence.
Understanding the Range of Instruments and Techniques
Chemistry experiments require proficiency with qualitative and quantitative tools that enable accurate data collection and analysis. Students are expected to use instruments safely, appropriately, and effectively, showing independence in applying them to varied contexts.
Importance of Instrumental Proficiency
Practical competence ensures that results are reproducible, reliable, and valid, aligning with scientific methodology. The ability to handle instruments correctly reflects understanding of both theoretical principles and experimental design.
Key purposes include:
Generating accurate and precise measurements.
Supporting chemical theory through empirical evidence.
Demonstrating control over variables and reducing experimental errors.
Gaining familiarity with industry-standard laboratory practice.
Measurement Instruments in Chemistry
Modern laboratories rely on a variety of measurement tools that provide quantitative data essential for analysis and evaluation.
Balances
Balances are used to measure mass with high precision. Analytical balances can detect differences of 0.001 g or smaller. They must be kept on stable surfaces, calibrated regularly, and used with care to prevent contamination.
Common practices:
Ensure the balance is level and zeroed before use.
Never place hot or reactive substances directly on the pan.
Record readings to the correct number of significant figures.
Thermometers and Temperature Probes
Temperature affects reaction rates and equilibrium, making accurate temperature measurement essential. Digital thermometers and probes linked to data loggers enable continuous monitoring.
Advantages of probes:
Greater precision and responsiveness than traditional mercury thermometers.
Digital data collection reduces human reading error.
Volumetric Equipment
Precise volume measurement is fundamental in analytical chemistry. Each device serves a distinct function, requiring correct handling techniques.
Burettes, Pipettes and Volumetric Flasks
These are essential for volumetric analysis and solution preparation.
Best practices:
Rinse with the solution to be used to prevent dilution errors.
Read the meniscus at eye level.
Use a pipette filler for safety, never mouth pipette.
Ensure the volumetric flask is clean, dry, and filled to the calibration mark.
Meniscus: The curved surface of a liquid in a container, read at the lowest point for accurate measurement.
Volumetric precision ensures reproducibility between titrations and calculations based on molar relationships.
Heating and Cooling Techniques
Temperature control enables chemists to influence reaction rates, product yields, and purity.
Common Heating Methods
Water baths – maintain a steady temperature below 100°C.
Sand baths – used for heating glassware evenly at higher temperatures.
Electric heaters – provide controlled, flameless heating for flammable substances.
Cooling and Condensation
For reactions under reflux, condensers prevent loss of volatile components by condensing vapours back into liquid form. Cooling may also be achieved using ice baths to slow exothermic reactions.
Reflux: The continuous boiling and condensation of a reaction mixture to prevent loss of volatile substances while maintaining a constant temperature.
Heating under reflux allows a reaction to be boiled while vapours condense and return to the flask, preventing loss of solvent or product.
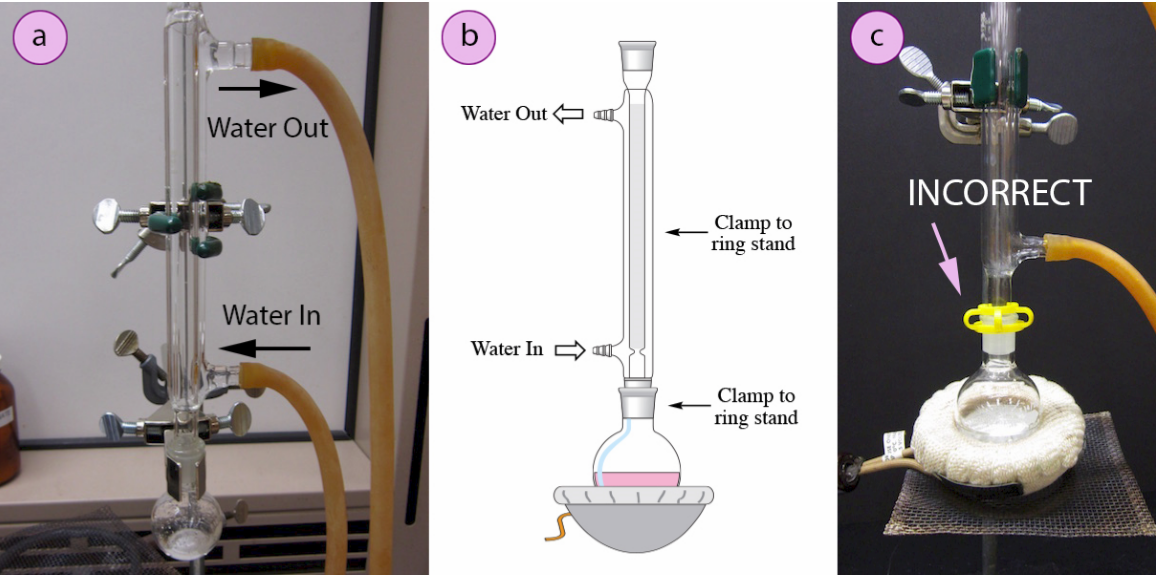
Reflux apparatus with vertical condenser and correct cooling-water direction. The setup enables prolonged heating without solvent loss and illustrates safe clamping points. The visible arrows for water flow match best laboratory practice described in the syllabus-aligned notes. Source
Separation and Purification Instruments
Purity is vital for reliable results. Techniques must isolate desired products while removing impurities efficiently.
Filtration
Used to separate insoluble solids from liquids. Variants include:
Gravity filtration with fluted paper for clarity.
Vacuum filtration for rapid separation using a Büchner funnel and flask.
Distillation
Separates liquids based on differing boiling points. Fractional distillation incorporates a column to enhance separation of components with close boiling points.
Recrystallisation
Purifies solids by dissolving in hot solvent and cooling to form pure crystals. Equipment includes beakers, filter funnels, and drying ovens.
Analytical Techniques and Instrumentation
Analytical chemistry relies on techniques that provide qualitative and quantitative data to identify and measure chemical substances.
Chromatography
Thin Layer Chromatography (TLC) and Paper Chromatography separate mixtures based on component solubility and adsorption.
Key equipment includes:
Stationary phase (TLC plate or paper).
Mobile phase (solvent).
Developing chamber and UV lamp for visualisation.
In TLC, the retention factor (R_f) is defined as distance moved by the centre of the spot divided by distance moved by the solvent front.
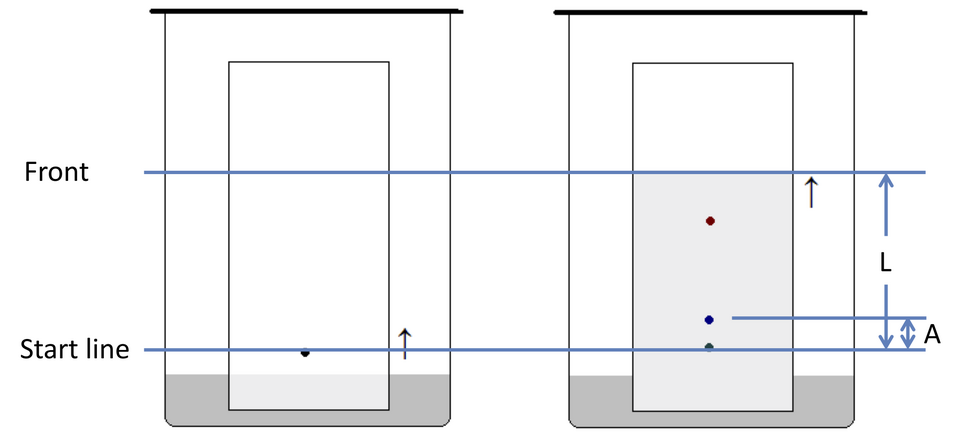
Labeled TLC plate depicting the baseline, sample spot, solvent front, and path lengths used to calculate Rf. The diagram focuses on essentials without clutter, ideal for A-level learning. Note: the figure also labels multiple example spots to illustrate separation, which slightly exceeds the minimal definition. Source
Retention Factor (Rf) = Distance moved by substance ÷ Distance moved by solvent front
Rf (no unit) = relative measure of mobility in chromatography
Spectroscopic Instruments
Modern laboratories use Infrared (IR) Spectroscopy and Mass Spectrometry (MS) to identify compounds.
IR Spectroscopy: Detects molecular vibrations and functional groups.
Mass Spectrometry: Determines molecular mass and structure through ionisation and mass-to-charge ratio (m/z) analysis.
Mass-to-Charge Ratio (m/z): The ratio of ion mass to its charge, used to identify species in a mass spectrometer.
Electrochemical and Data Analysis Equipment
Electrochemical methods and digital tools are increasingly vital for monitoring chemical change.
Electrochemical Cells
Used to measure electrode potentials and study redox reactions. Voltmeters record cell potential accurately. Electrodes must be cleaned and assembled under safe conditions.
A simple electrochemical cell uses two half-cells linked by a salt bridge; the measured cell potential (E_cell) depends on electrode potentials and temperature.
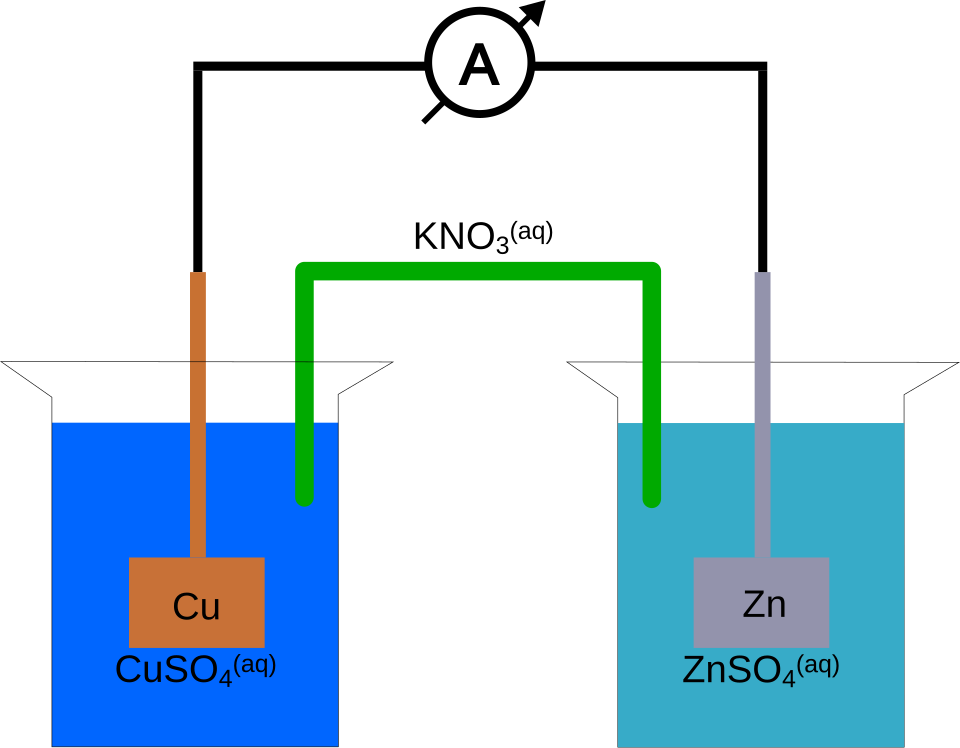
Annotated galvanic cell (Zn|ZnSO₄ ∥ KCl salt bridge ∥ CuSO₄|Cu) with voltmeter connections. The diagram highlights the function of the salt bridge and electron flow direction, supporting instrument setup and measurement notes. Labels closely match terminology used in A-level practical skills. Source
Safety considerations:
Avoid direct contact with corrosive electrolytes.
Dispose of heavy metal solutions according to chemical waste regulations.
Digital and Computer-Based Tools
Software tools process experimental data efficiently and improve accuracy. Data loggers and spreadsheet programs enable real-time monitoring and graphical representation.
Safe and Responsible Laboratory Practice
Effective use of equipment must always be accompanied by risk assessment and adherence to health and safety regulations.
Best practice guidelines:
Identify hazards (e.g., flammable, toxic, corrosive substances).
Use personal protective equipment (lab coats, goggles, gloves).
Label all chemicals and apparatus correctly.
Dispose of waste following environmental and safety protocols.
Risk Assessment: A systematic process of identifying hazards, evaluating potential risks, and implementing control measures to ensure safe practice.
Students must demonstrate independence, accuracy, and responsibility when handling instruments — essential qualities for both assessment and real-world laboratory competence.
FAQ
Precision refers to the consistency of repeated measurements, while accuracy describes how close a measurement is to the true or accepted value.
For example, a balance may provide precise results if readings are consistent, but those readings may not be accurate if the instrument is miscalibrated.
Reliability is ensured through regular calibration, use of control samples, and repetition of measurements.
Other good practices include:
Cleaning and maintaining equipment before each use.
Using appropriate glassware for the required precision.
Recording all data with correct significant figures.
Changing the solvent can alter the polarity and therefore change how substances interact with the stationary phase.
To compare results between runs:
Use the same solvent mixture and TLC plate type.
Maintain consistent temperature and development distance.
This ensures reproducibility and accurate identification of compounds.
Always conduct experiments in a fume cupboard to prevent inhalation of vapours. Keep all ignition sources away since many solvents are flammable.
Additional precautions include:
Wearing appropriate eye protection and gloves.
Using clamps to secure apparatus.
Avoiding overfilling the reaction flask to reduce risk of boiling over.
Digital data loggers record continuous data automatically, reducing human error from reading analog scales.
They offer advantages such as:
Higher measurement resolution and consistency.
Automatic averaging of multiple readings.
Easy graphing and analysis of temperature, pH, or voltage trends over time.
Practice Questions
A student sets up a reflux apparatus to heat a reaction mixture. Explain why water enters the condenser at the bottom and leaves at the top. (2 marks)
1 mark: States that water enters at the bottom so the condenser remains full of water.
1 mark: Explains that this ensures efficient cooling and condensation of vapours back into the reaction flask.
A student uses thin layer chromatography (TLC) to analyse a mixture of organic compounds. The solvent front moves 6.0 cm from the baseline, and a spot moves 3.6 cm. The student identifies two additional faint spots on the plate.
(a) Calculate the Rf value for the main spot. (1 mark)
(b) Explain what the Rf value represents. (2 marks)
(c) Suggest two reasons why faint additional spots might appear. (2 marks)
(5 marks)
(a) 1 mark: Correct calculation: Rf = 3.6 / 6.0 = 0.60.
(b) 1 mark: States that Rf represents the ratio of distance moved by substance to distance moved by solvent front.
1 mark: Indicates that it reflects the substance’s affinity for stationary and mobile phases.(c) 1 mark: Impurity or contamination of the sample.
1 mark: Overloading or incomplete separation during development.

