OCR Specification focus:
'Apply investigative approaches and methods to practical work, including problem solving in practical contexts, demonstrating independence.'
Investigative Approaches and Independent Thinking
Students undertaking OCR A-Level Chemistry must develop independent investigative skills that allow them to plan, conduct, and evaluate experiments confidently. These skills form the foundation of scientific inquiry, enabling students to think critically, solve problems, and make decisions when faced with unexpected results or practical challenges.
Understanding Investigative Approaches
Investigative approaches in chemistry involve systematically exploring a scientific question through observation, hypothesis testing, experimentation, and analysis. They encourage curiosity, accuracy, and logical reasoning—skills central to all scientific disciplines.
The Scientific Method
The scientific method underpins all investigative work in chemistry. It follows a structured process:
Observation – identifying patterns, anomalies, or questions about chemical systems.
Hypothesis formation – proposing a testable explanation based on chemical principles.
Experimentation – designing and performing practical work to test the hypothesis.
Analysis and evaluation – interpreting results, identifying errors, and suggesting improvements.
Each stage demands independent thought and clear scientific reasoning.
Investigations move iteratively through observation, hypothesis, testing, analysis and evaluation.
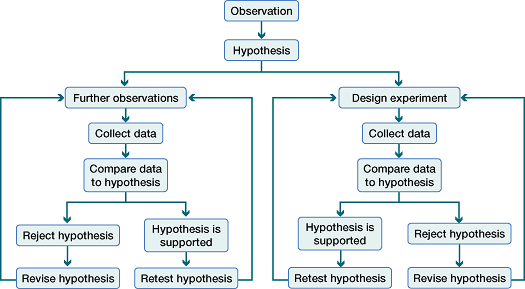
A clear flowchart of the scientific method emphasising that inquiry is cyclical, not linear. Arrows show how questions, testing, and analysis feed back into revised hypotheses. This supports independent planning and evaluation during practical work; extra detail beyond the syllabus is minimal. Source
Developing Independent Thinking
Independent thinking in practical chemistry means taking responsibility for how investigations are planned and conducted. It goes beyond simply following a set of instructions.
Characteristics of Independent Learners
Initiative: Choosing suitable methods, equipment, and materials for the investigation.
Critical judgement: Assessing whether data are reliable, valid, and sufficient.
Adaptability: Responding effectively when results deviate from expectations.
Reflection: Evaluating both success and limitations of experimental design.
Independent thinking is vital in problem-solving, where students must analyse issues logically and draw on their theoretical understanding to propose realistic solutions.
Planning and Designing Investigations
Careful planning is central to effective practical chemistry. Planning involves converting a research question into a testable investigation.
Steps in Planning
Define the research question – state clearly what is being investigated.
Formulate a hypothesis – predict the relationship between variables.
Identify variables – distinguish between independent, dependent, and controlled variables.
Select appropriate apparatus and methods – ensure accuracy and safety.
Plan data collection – determine what measurements to record and how often.
Identify potential hazards – apply safe laboratory practices.
Variables and Control
Independent Variable: The variable that is deliberately changed during an experiment to observe its effect.
Dependent Variable: The variable that is measured to determine the effect of the change.
Controlled Variables: Factors kept constant to ensure that any change in the dependent variable is due only to the independent variable.
Ensuring effective control of variables allows reliable and reproducible results, forming the basis of valid scientific conclusions.
Problem Solving in Practical Contexts
Problem solving requires combining chemical knowledge with practical reasoning. When unexpected outcomes occur—such as anomalous data, equipment failure, or contamination—students should apply logical strategies to identify and rectify the issue.
Approaches to Problem Solving
Re-examine procedural steps for potential human or systematic errors.
Check instrument calibration or repeat measurements for consistency.
Consider chemical reasoning – e.g. incomplete reactions, side reactions, or impurities.
Suggest and implement methodological improvements.
Problem solving also includes analysing why an experiment did or did not confirm the hypothesis and using evidence to support scientific reasoning.
Recording and Evaluating Observations
Accurate recording of observations and measurements is fundamental. Students must maintain detailed records in lab notebooks or digital logs, including:
Date, title, and purpose of the experiment.
Method, materials, and conditions used.
Qualitative observations (colour, odour, precipitate formation).
Quantitative data with correct units and precision.
Accuracy describes closeness to a true value, whereas precision describes the spread of repeated measurements.
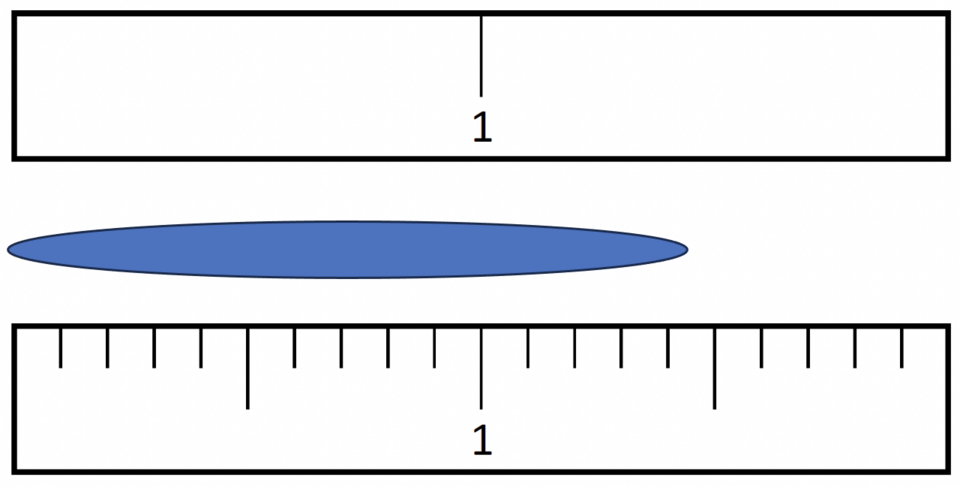
Diagram comparing two rulers to show how finer scale divisions yield more significant figures and greater precision. This clarifies why instrument selection is a key investigative decision. The image focuses on measurement quality; any broader metrology context is intentionally minimal. Source
Evaluating Data Quality
After data collection, evaluation ensures reliability and validity:
Reliability – consistency of results when repeated.
Accuracy – closeness to the true value.
Precision – consistency between replicate readings.
Validity – whether the investigation truly tests the hypothesis.
Drawing Conclusions and Communicating Findings
Interpreting results demands connecting data to theoretical chemistry. A sound conclusion:
Directly addresses the hypothesis.
Discusses whether results support or refute it.
Acknowledges sources of uncertainty and limitations.
Suggests further research or improvements.
Effective communication of findings demonstrates scientific literacy—the ability to interpret evidence and articulate reasoning clearly using appropriate terminology and units.
The Role of Reproducibility
Reproducibility is essential for verifying scientific results. For findings to be reproducible:
Experiments must include sufficient procedural detail for others to replicate them.
Equipment calibration and standardised methods should be maintained.
Raw data should be retained for peer verification.
Reproducibility not only validates experimental outcomes but also builds confidence in the reliability of chemical research.
Ethical and Responsible Investigation
Independent investigation also carries an ethical dimension. Chemists must ensure that all experiments are:
Conducted safely with regard to people and the environment.
Reported honestly, without falsification or selective omission of data.
Designed to minimise waste and pollution.
These practices align with professional scientific integrity and environmental responsibility.
Linking Practical Work to Theory
Applying investigative approaches allows theoretical understanding to be tested through observation. For example, investigating the effect of concentration on reaction rate links directly to collision theory and rate equations. Independent thinking ensures that such investigations deepen conceptual understanding rather than merely confirm known results.
Skills Demonstrated in the Practical Endorsement
Through investigative and independent work, OCR Chemistry students are expected to show that they can:
Design and implement a coherent scientific investigation.
Identify problems and make justified adjustments.
Analyse data critically and communicate conclusions clearly.
Demonstrate autonomy and creativity in problem solving.
These competencies reflect the transition from guided experimentation to genuine scientific inquiry—a core objective of A-Level practical chemistry.
A researcher writes in a bound lab notebook, exemplifying good practice for transparent methods, raw data, and decision trails. This visual underlines accountability and independence in investigations. No extraneous detail beyond the syllabus focus is included. Source
FAQ
An independent investigation typically begins with defining a clear research question, followed by forming a testable hypothesis. The next stage involves identifying independent, dependent, and controlled variables.
Students then plan the method, including the choice of apparatus and safety measures. Data collection, analysis, and evaluation follow, allowing conclusions to be drawn based on evidence. Each stage should demonstrate the student’s independent reasoning and understanding of chemical principles.
Independent thinking can be demonstrated by:
Re-examining the procedure and identifying possible sources of error.
Adjusting experimental conditions to improve reliability.
Explaining results using chemical reasoning rather than repeating the same method blindly.
Proposing method improvements that align with scientific logic.
These actions show initiative and understanding beyond following instructions.
Recording observations immediately ensures accuracy and prevents data loss. Delays may lead to forgotten details or unintentional bias in recollection.
It also provides a reliable time-based record, useful for identifying trends or anomalies. For example, colour changes or gas evolution may occur rapidly and need prompt documentation to maintain valid experimental evidence.
Reliability can be evaluated by:
Repeating measurements and comparing results for consistency.
Using appropriate apparatus with suitable precision.
Identifying and reducing random errors.
Checking for reproducibility across different trials or peers.
High reliability indicates the method and measurements are consistent, even if some uncertainty remains.
To develop independence, students should:
Plan investigations from hypothesis to analysis without excessive teacher guidance.
Make informed choices about apparatus, measurement techniques, and safety.
Reflect critically on data quality and suggest logical improvements.
Research background theory using reliable scientific sources.
These strategies encourage confidence, initiative, and professional-level scientific thinking.
Practice Questions
During an investigation, a student records multiple readings of temperature using the same thermometer. Explain the difference between accuracy and precision in this context. (2 marks)
1 mark for stating that accuracy refers to how close a measurement is to the true or accepted value.
1 mark for stating that precision refers to how close repeated measurements are to each other, regardless of whether they are accurate.
A student investigates how the concentration of hydrochloric acid affects the rate of reaction with magnesium ribbon. The student independently plans and conducts the experiment.
(a) Identify three variables the student should control to ensure a valid test. (3 marks)
(b) Describe two ways the student could demonstrate independent thinking during this investigation. (2 marks)
(5 marks)
(a)
1 mark for controlling the length or surface area of magnesium ribbon.
1 mark for controlling the temperature of the reaction mixture.
1 mark for controlling the volume of acid used (or total solution volume).
(b)
1 mark for modifying the experimental method based on unexpected results or observations.
1 mark for analysing data and evaluating errors to suggest improvements to the procedure.

