OCR Specification focus:
‘Measure pH using pH charts, a pH meter, or a pH probe connected to a data logger, as appropriate to the investigation.’
Measuring and Controlling pH
Introduction (25 words)
Understanding how to measure and control pH accurately is essential for ensuring reliable experimental results, as pH affects reaction rates, equilibria, and the stability of chemical species.
Understanding pH
The pH of a solution indicates its hydrogen ion concentration, which determines its acidity or alkalinity. Precise pH measurement is crucial for analytical, synthetic, and biological experiments.
pH: A logarithmic measure of hydrogen ion concentration, defined as pH = –log₁₀[H⁺].
Even small changes in pH can significantly alter reaction outcomes, making accurate measurement and control vital in laboratory practice.
Methods of Measuring pH
OCR requires students to be able to measure pH using pH charts, pH meters, or pH probes connected to data loggers. Each method varies in accuracy, sensitivity, and application.
pH Indicators and Charts
pH indicators are weak acids or bases that change colour depending on the pH of the solution. They are simple, inexpensive, and useful for approximate measurements.
Common pH Indicators:
Litmus: Red in acid, blue in alkali (approximate range pH 5–8)
Methyl orange: Red in acid, yellow in alkali (range pH 3.1–4.4)
Phenolphthalein: Colourless in acid, pink in alkali (range pH 8.3–10.0)
Advantages:
Quick and easy to use.
No calibration required.
Limitations:
Subjective interpretation of colour.
Limited precision (±0.5 pH units).
Unsuitable for coloured or opaque solutions.
pH charts accompany universal indicator papers, providing a colour scale typically ranging from pH 1 to 14. Comparing the paper colour to the chart gives an approximate pH value.

A universal indicator colour scale for estimating pH by comparison with the colour of indicator paper or solution. This supports rapid, approximate pH readings where high precision is not required. The image shows coloured standards from strongly acidic to strongly alkaline solutions. Source
Using a pH Meter
pH meters provide quantitative and highly accurate pH readings. A pH meter measures the potential difference between two electrodes and converts this into a pH value.
pH Meter: An instrument that determines the hydrogen ion activity in a solution through an electrochemical measurement.
Components of a pH Meter:
Glass electrode (sensitive to [H⁺] ions)
Reference electrode (provides a constant potential)
Digital readout/display unit
Operating Principles:
The glass electrode develops a potential difference proportional to the hydrogen ion concentration. This voltage difference is converted into a pH value by the meter’s internal circuitry.
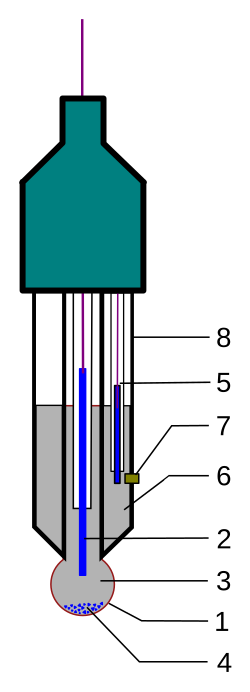
Annotated diagram of a combined glass pH electrode used with pH meters. Labels identify the glass bulb, reference electrode, junction, and internal solutions, clarifying how the electrochemical cell generates a voltage read as pH. This includes structural detail beyond the syllabus but directly supports understanding of meter operation. Source
Calibration:
Before use, a pH meter must be calibrated with standard buffer solutions (commonly pH 4.00, 7.00, and 10.00) at room temperature to ensure accuracy. Calibration corrects for electrode drift and temperature variations.
Good Practice for Calibration:
Rinse electrodes with distilled water between measurements.
Avoid contamination of buffer solutions.
Store electrodes in an appropriate storage solution, not dry.
Advantages:
Accurate to ±0.01 pH units.
Suitable for a wide range of sample types.
Digital readout minimises human error.
Limitations:
Requires regular calibration and maintenance.
Fragile glass electrode can break.
Readings may drift if the probe is contaminated.
Using a pH Probe with a Data Logger
Modern laboratories often use pH probes connected to data loggers, allowing continuous monitoring and recording of pH changes during reactions.
A benchtop pH probe connected to a LabQuest interface for continuous, time-stamped data logging. Such setups support real-time pH monitoring during titrations or kinetic investigations, aligning with OCR’s requirement to use a pH probe with a data logger. Extra contextual details (e.g., the specific LabQuest interface) are shown but remain within scope. Source
Data Logger: An electronic device that automatically records data over time using sensors or probes.
Benefits of Data Logging:
Provides real-time monitoring of pH changes.
Enables graphical analysis of reaction rates and equilibria.
Reduces manual recording errors.
Data loggers are particularly useful in kinetic experiments and titration curves, where precise, time-dependent pH variation is analysed.
Controlling pH
In many reactions, maintaining a constant pH is essential. This is achieved using buffers, neutralisation techniques, or automated titration systems.
Buffer Solutions
Buffer Solution: A solution that resists changes in pH when small amounts of acid or base are added.
Buffers typically consist of a weak acid and its conjugate base, or a weak base and its conjugate acid. They maintain pH stability by reacting with added hydrogen or hydroxide ions.
Common Buffer Systems:
Ethanoic acid and sodium ethanoate (acidic buffer, pH ≈ 4.75)
Ammonium chloride and ammonia (basic buffer, pH ≈ 9.25)
Applications:
Biological systems (e.g. maintaining blood pH around 7.4)
Analytical chemistry (e.g. chromatography and electrophoresis)
Industrial processes (e.g. fermentation control)
Factors Affecting pH Measurement Accuracy
Accurate pH measurement depends on several controlled factors:
Temperature
Temperature affects electrode potential and dissociation equilibria. Most pH meters have automatic temperature compensation (ATC) to correct readings.
Ionic Strength
Highly concentrated or non-aqueous solutions may cause electrode interference, requiring specialised electrodes.
Contamination
Residue on electrodes or in buffer solutions leads to systematic errors. Always rinse thoroughly and handle carefully.
Electrode Maintenance
Over time, electrodes deteriorate. Regular replacement and proper storage prolong lifespan and maintain accuracy.
Safety and Good Laboratory Practice
When measuring and controlling pH, it is vital to handle acids, bases, and probes safely:
Wear eye protection and gloves when handling corrosive or irritant substances.
Use fume cupboards for volatile acids or bases.
Dispose of acidic and basic waste appropriately.
Ensure electrical equipment like pH meters are dry and intact before use.
Clean up spillages immediately, neutralising acids with sodium hydrogen carbonate and alkalis with dilute acid.
These precautions align with OCR’s emphasis on safe handling of equipment and chemicals during practical investigations.
Summary of Key Techniques for OCR Practical Endorsement
Students should demonstrate proficiency in:
Selecting an appropriate pH measurement technique for the investigation.
Correctly calibrating and using a pH meter.
Recording and interpreting digital data accurately.
Managing experimental risk and ensuring chemical safety.
Maintaining accurate and reliable records of pH measurements.
Accurate and safe measurement of pH underpins the reliability of practical chemistry, fulfilling the OCR requirement to measure pH using pH charts, a pH meter, or a pH probe connected to a data logger, as appropriate to the investigation.
FAQ
Temperature influences both the dissociation of acids and the electrode potential of pH probes. As temperature increases, the mobility of ions changes, slightly altering the measured pH.
Most modern pH meters feature automatic temperature compensation (ATC), which adjusts the reading to standard conditions. Without ATC, readings at high or low temperatures can deviate by up to 0.1–0.3 pH units.
The glass membrane on a pH electrode must stay hydrated to function correctly. When dry, it loses its selective permeability to hydrogen ions, leading to inaccurate or slow readings.
Store electrodes in a specialised storage solution or pH 4 buffer, not distilled water, which can leach ions from the glass and damage the electrode.
Calibration adjusts the instrument to read correctly using standard buffers, while standardisation verifies that the calibration remains valid over time.
Good laboratory practice includes:
Calibrating the pH meter at least daily.
Standardising weekly or when accuracy is critical. This ensures measurement reliability across multiple experiments.
A pH probe is often a combined electrode integrated into a sealed unit, suitable for data logging or field work.
Chemists choose it when:
Continuous pH tracking is required (e.g. titrations, reaction monitoring).
Minimal setup time or portability is needed.
Traditional electrodes may offer greater customisation but require more maintenance and calibration steps.
Buffers are effective only within about one pH unit of their pKa value. Beyond this range, they lose their ability to resist changes in acidity or alkalinity.
Additionally, buffers can be:
Temperature-sensitive, as equilibrium shifts affect their balance.
Dilution-sensitive, since lower concentration reduces buffering capacity.
Chemically reactive, especially if components interact with reagents in the experiment.
Practice Questions
A student uses universal indicator paper to estimate the pH of an aqueous solution. The paper turns orange when dipped into the solution.
(a) Estimate the pH range of the solution.
(b) State one reason why this method is less accurate than using a pH meter.
(2 marks)
(a)
Correctly identifies pH range around 3–4 (accept 3–5) – 1 mark
(b)
Explains that colour interpretation is subjective or that the method gives only approximate values (±0.5 pH units) – 1 mark
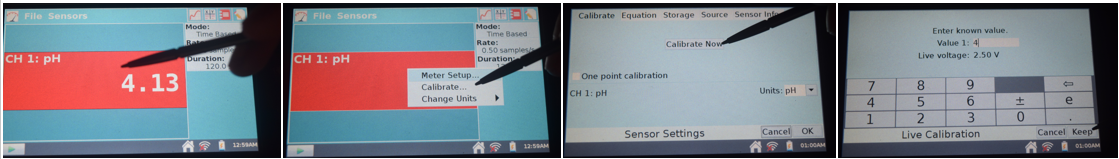
A chemist is monitoring the progress of a reaction by measuring the pH at regular intervals using a pH probe connected to a data logger.
(a) Explain why a pH probe and data logger are more suitable for this experiment than using a pH meter manually. (2 marks)
(b) State two steps required to calibrate the pH probe before use. (2 marks)
(c) Suggest one reason why the pH readings might drift during the experiment and how this could be corrected. (1 mark)
(5 marks)
(a)
Allows continuous monitoring of pH changes during the reaction – 1 mark
Reduces human error and provides automatic data recording/graphing – 1 mark
(b)
Any two of the following:
Rinse the probe with distilled water between calibration solutions – 1 mark
Use standard buffer solutions (e.g. pH 4, 7, 10) – 1 mark
Adjust the meter to match the known pH values of the buffers – 1 mark
(Max 2 marks)
(c)
pH readings may drift due to contaminated or dried electrode – 1 mark
Corrected by cleaning or rehydrating the probe in storage solution – award if both cause and remedy mentioned – 1 mark (max)

