OCR Specification focus:
‘Perform qualitative tests for ions and organic functional groups; use thin layer or paper chromatography to separate and identify substances.’
Analytical identification techniques form a core set of skills in A-Level Chemistry, enabling students to detect ions, recognise organic functional groups, and separate mixtures using chromatography with precision and scientific accuracy.
Qualitative Analysis of Inorganic Ions
Analytical identification often begins with qualitative inorganic tests, essential for determining the presence of cations and anions in unknown samples through characteristic observations.
Cation Tests
A number of cations produce distinctive reactions when treated with aqueous sodium hydroxide or ammonia.
Flame tests help identify Group 1 and Group 2 metal ions by characteristic flame colours.
Precipitation reactions with NaOH allow identification of transition metal ions based on precipitate colour changes.
Reaction with NH₃(aq) may dissolve certain precipitates due to complex ion formation.
Complex Ion: A species formed when a central metal ion binds to surrounding ligands via coordinate bonds.
These observations rely on oxidation state stability and ligand–metal interactions, reinforcing understanding of transition metal chemistry.
Anion Tests
Anions can be distinguished by specific reagents that produce precipitates or gas evolution.
Carbonates release carbon dioxide when treated with acids.
Halide ions react with acidified silver nitrate to form precipitates with distinct colours:
Chloride: white
Bromide: cream
Iodide: yellow
Sulfate ions produce a white precipitate when exposed to barium chloride in the presence of acid.
Halide ions are identified by adding acidified silver nitrate and observing the colour of the silver halide precipitate: white for chloride, cream for bromide and yellow for iodide.

Test tubes showing the precipitates formed when chloride, bromide and iodide ions react with silver nitrate: white AgCl, cream AgBr and pale yellow AgI. Source
Between definition blocks, students should understand that these characteristic reactions arise from differences in solubility product and ligand exchange behaviour.
Identification of Organic Functional Groups
Organic qualitative analysis relies on targeted tests that exploit the chemical reactivity of functional groups.
Tests for Key Functional Groups
Organic molecules can often be identified quickly using colour changes or observations linked to specific reagents.
Aldehydes are oxidised by Tollens’ reagent, producing a silver mirror as the aldehyde is converted into a carboxylate ion.
Ketones do not react with Tollens’, helping distinguish them from aldehydes.
Alcohols may be classified using acidified potassium dichromate, where primary and secondary alcohols cause the orange solution to turn green upon oxidation.
Alkenes are identified by the decolourisation of bromine water, showing the presence of a carbon–carbon double bond.
Carboxylic acids effervesce with carbonates due to CO₂ release.
Oxidation (Organic Context): A reaction involving the increase in the number of carbon–oxygen bonds or decrease in carbon–hydrogen bonds.
Organic qualitative tests hinge on functional group reactivity, making them useful for rapid characterisation.
Chromatographic Identification Techniques
The OCR specification emphasises the ability to use thin layer chromatography (TLC) and paper chromatography to separate and identify substances.
Principles of Chromatography
Chromatography separates components of a mixture based on differences in affinity between the stationary phase and mobile phase.
Stationary Phase: The solid or liquid phase that remains fixed in place during chromatography, allowing separation based on interactions with analytes.
A sentence between blocks reinforces understanding: separation efficiency depends on polarity, solubility and intermolecular interactions, determining how far each component travels.
Mobile Phase: The solvent or mixture of solvents that moves through the stationary phase, carrying analytes at varying rates.
In both paper chromatography and thin layer chromatography (TLC), a solvent mobile phase rises up the stationary phase by capillary action, carrying dissolved sample components.
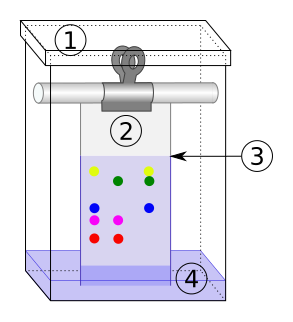
Diagram of a chromatography tank showing the paper as the stationary phase and the solvent as the mobile phase as it rises to form a solvent front. Source
Setting Up TLC and Paper Chromatography
Students must be able to set up chromatography safely and correctly.
Use a pencil to mark the baseline to avoid ink contamination.
Apply small, concentrated sample spots using capillary tubes.
Place the chromatogram in a beaker with a shallow layer of solvent, ensuring the baseline is above solvent level.
Cover the beaker to maintain a saturated atmosphere and promote even solvent rise.
Remove the plate once the solvent front nears the top and mark the solvent front immediately.
These steps ensure reliable separation and minimise analytical error.
Interpreting Chromatograms
Chromatography provides qualitative identification through Rf values and comparison with known standards.
Distinct spots at characteristic Rf values can then be compared with known standards to identify the components in an unknown mixture.
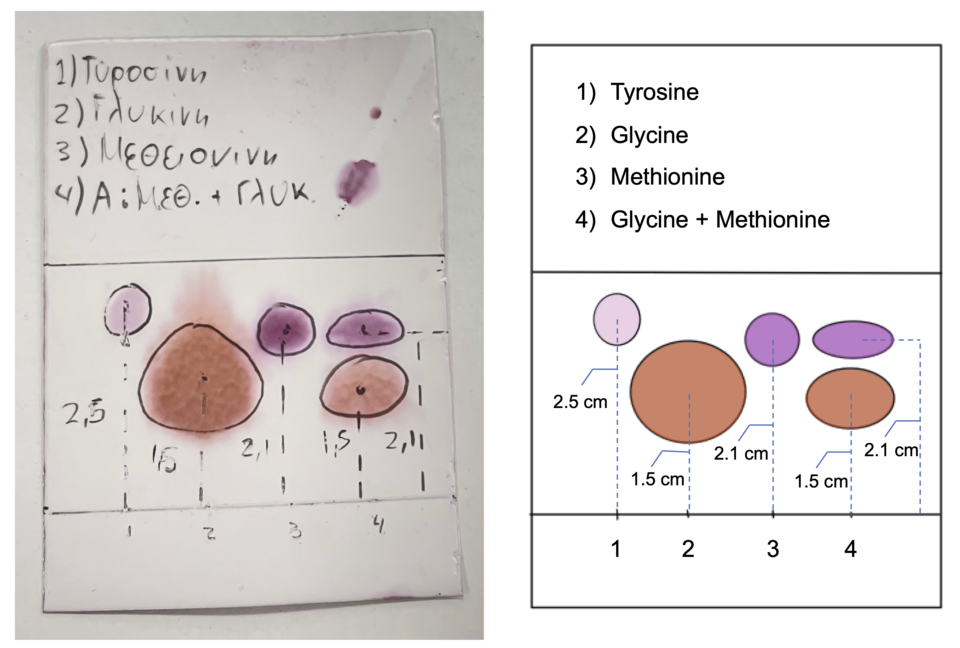
Thin layer chromatogram of amino acids showing distinct spots for several standards and an unknown sample for comparison. Source
Retention Factor (Rf) = Distance travelled by substance ÷ Distance travelled by solvent front
Distance travelled by substance = Measurement from baseline to centre of spot (cm)
Distance travelled by solvent front = Measurement from baseline to solvent front (cm)
Rf values lie between 0 and 1 and allow comparison to reference data.
A substance with stronger attraction to the stationary phase will travel a shorter distance, giving a lower Rf.
Co-spotting with known samples strengthens identification confidence.
After using the equation, it is crucial to note that experimental conditions such as solvent polarity and temperature must remain consistent when comparing Rf data.
Safety and Good Practice in Analytical Testing
Analytical identification requires strict adherence to laboratory safety.
Wear eye protection and use fume cupboards for volatile organic reagents.
Handle oxidising agents, such as dichromate, with care.
Dispose of silver compounds responsibly due to environmental toxicity.
Ensure chromatographic solvents are kept away from naked flames, as many are flammable.
These practices support safe, effective analytical work in accordance with the OCR practical endorsement expectations.
FAQ
Some transition metal precipitates appear visually similar, so secondary tests are used to confirm identity.
Adding excess sodium hydroxide or aqueous ammonia can reveal differences in solubility or complex formation:
Copper(II) hydroxide dissolves in excess ammonia to form a deep blue complex.
Chromium(III) hydroxide dissolves in excess hydroxide to form a green chromite ion.
Iron(III) hydroxide remains insoluble in both reagents.
Observing changes over time can also help, as some precipitates darken due to air oxidation.
Acidification removes interfering anions that would otherwise produce misleading precipitates.
Carbonate and hydroxide ions can form silver carbonate or silver oxide, giving false positives. Adding dilute nitric acid neutralises these ions without reacting with the halides themselves.
Acidification also improves reliability by ensuring the silver nitrate reacts only with chloride, bromide, or iodide ions.
Rf values are only meaningful when experimental conditions are consistent.
Important variables to control include:
Solvent composition, as polarity strongly affects migration.
Saturation of the chromatography chamber.
Type and thickness of the stationary phase.
Temperature, which influences solubility and diffusion.
Maintaining these conditions ensures Rf comparisons between plates are valid.
TLC plates often contain a fluorescent indicator that allows spots to be visualised under UV, but not all compounds absorb UV radiation.
Compounds lacking conjugated systems may not quench the fluorescence, making the spots invisible. In these cases, alternative visualisation methods such as iodine vapour, KMnO4 staining, or ninhydrin (for amino acids) are used.
The visualisation method chosen must be appropriate for the functional groups present.
Co-elution occurs when two substances travel the same distance and appear as a single spot.
To reduce this risk:
Modify the solvent system by increasing or decreasing polarity.
Use mixed-solvent systems for finer control.
Switch to a different stationary phase such as silica instead of alumina.
Adjust the development distance to improve spread between components.
These adjustments help achieve better resolution and more reliable identification.
Practice Questions
A student adds acidified silver nitrate solution to a sample containing an unknown halide ion. A cream precipitate forms.
(a) Identify the halide ion present. (1 mark)
(b) State the ionic equation for the formation of this precipitate. (1 mark)
(2 marks)
(a)
Bromide ion / Br⁻ (1 mark)
(b)
Ag⁺(aq) + Br⁻(aq) → AgBr(s) (1 mark) (Allow any correct ionic equation showing formation of solid silver bromide.)
A mixture of three organic compounds is analysed using thin layer chromatography (TLC).
The plate is developed using an appropriate solvent. After development, four spots are visible: three pure reference standards (A, B and C) and one spot from the mixture.
(a) Explain why the baseline must be drawn in pencil rather than ink. (1 mark)
(b) State two factors that affect how far a substance travels on a TLC plate. (2 marks)
(c) The unknown sample has the same Rf value as reference compound B. Explain how the student could increase confidence that the unknown contains compound B. (2 marks)
(5 marks)
(a)
Pencil is insoluble in the solvent / ink would dissolve and interfere with the chromatogram (1 mark)
(b) Any two of:
Polarity of the substance (1 mark)
Interactions/affinity with the stationary phase (1 mark)
Solubility in the mobile phase (1 mark)
Strength of intermolecular forces between compound and phases (1 mark)
(c)
Any two of:
Co-spot the unknown with reference compound B on the same baseline (1 mark)
If both produce a single spot at the same height / same Rf under identical conditions, this confirms identity more reliably (1 mark)
(Allow equivalent wording.)

