OCR Specification focus:
‘Set up electrochemical cells and measure voltages; handle corrosive, irritant, flammable and toxic substances carefully; measure reaction rates by initial-rate and continuous methods.’
Electrochemical cells, safe chemical handling, and methods for measuring reaction rates form an essential practical foundation in A-Level Chemistry, supporting accurate experimentation, reliable data collection, and safe laboratory practice.
Electrochemical Cells
Electrochemical cells allow students to measure the electromotive force (EMF) produced when two half-cells are connected under standard or controlled conditions. This provides valuable insight into redox behaviour and practical applications of electrode potentials.
Key Components of an Electrochemical Cell
Two half-cells containing oxidation and reduction reactions
Metal electrodes or inert electrodes when ions are involved
Aqueous ionic solutions with known concentrations
A salt bridge, often potassium nitrate soaked into filter paper, to maintain charge balance
A high-resistance voltmeter to measure cell potential without drawing current
Constructing and Measuring a Cell Voltage
Students must be able to set up the apparatus safely and effectively:
Clean the metal electrodes before use to ensure consistent electron flow.
Immerse each electrode in an appropriate ionic solution (e.g., Zn(s) in Zn²⁺).
Insert the salt bridge securely between the two solutions without mixing them directly.
Connect electrodes to the voltmeter using crocodile clips, ensuring correct polarity.
Record the cell potential only when readings have stabilised.
Safe Handling of Chemicals
Practical electrochemical work may involve corrosive, irritant, flammable, or toxic substances, particularly acid solutions, metal ions, organic solvents, or redox reagents.

This set of Globally Harmonized System hazard pictograms shows the key symbols used to label corrosive, flammable, acute-toxicity and irritant chemicals commonly encountered in electrochemical experiments. These icons help students immediately identify risks when working with metal ions, acids, solvents and other reagents. The grid also includes additional pictograms not required in this subtopic but useful for wider chemical-safety context. Source
Understanding Hazard Symbols
Students must be able to recognise and respond to the following:
Corrosive: Can cause severe burns; common in strong acids and alkalis.
Irritant: Causes inflammation or discomfort upon contact.
Flammable: Easily ignited; often applies to organic solvents.
Toxic: Harmful or fatal if swallowed, inhaled or absorbed.
Risk Minimisation Strategies
Wear appropriate PPE: goggles, lab coat, and gloves where appropriate.
Work in a well-ventilated area or fume cupboard when handling volatile or toxic substances.
Store chemicals correctly, abiding by separation rules (e.g., acids away from organics).
Label all containers clearly before beginning any procedure.
Know the location of emergency equipment such as eye-wash stations and spill kits.
Risk Assessment: A structured evaluation of potential hazards and the steps required to minimise harm during an experiment.
Measuring Reaction Rates
This subsubtopic requires proficiency in initial-rate and continuous-monitoring methods for determining how quickly a chemical reaction proceeds.
Initial-Rate Method
The initial-rate method focuses on measuring the rate at the very start of a reaction, when concentrations have not yet changed substantially. Students typically use this approach when examining the effect of varying conditions such as concentration or temperature.
Rate of Reaction: The change in concentration of a reactant or product per unit time.
An initial rate can be determined by:
Measuring the time taken for a visible change (e.g., formation of a precipitate).
Taking the inverse of the time measured when the change is proportional to 1/time.
Plotting early data points and determining the gradient of the tangent at time zero.
A clear experimental design ensures that the time measurement corresponds directly to the change in concentration.
Continuous Monitoring Method
Continuous methods involve collecting data throughout the reaction rather than at a single point. This is particularly useful when following reactions that produce measurable physical changes, such as gas volume or colour intensity.
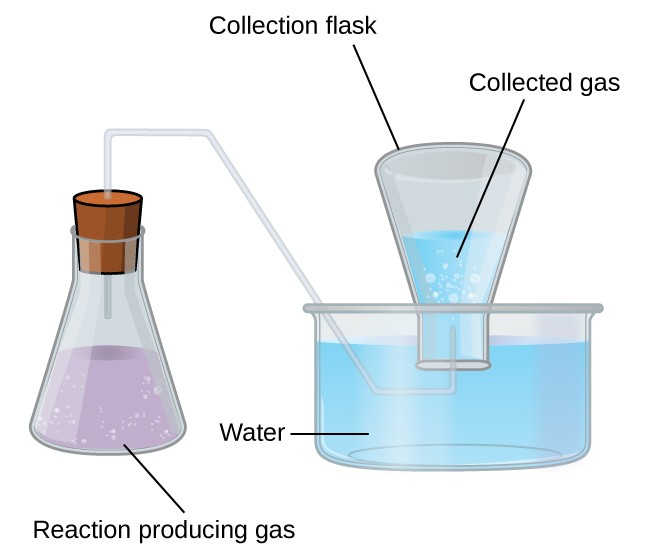
This diagram illustrates a reaction flask delivering gas into an inverted collection flask submerged in water, enabling continuous measurement of gas volume during a reaction. Tracking gas volume at timed intervals allows students to determine rate profiles. The surrounding textbook material includes gas-pressure concepts beyond the syllabus, but the apparatus itself matches OCR expectations. Source
Common continuous techniques include:
Gas collection using a gas syringe for reactions producing gaseous products.
Mass loss using a balance for reactions where gas escapes.
Colorimetry, which monitors changes in absorbance linked to concentration.
pH measurement for reactions involving strong acids or bases.
Beer–Lambert Law (A) = ε c l
A = Absorbance (no units)
ε = Molar absorptivity (dm³ mol⁻¹ cm⁻¹)
c = Concentration (mol dm⁻³)
l = Path length of cuvette (cm)
When applying continuous monitoring, students should ensure readings are taken at regular intervals and that the measuring equipment remains steady and properly calibrated.
Improving Accuracy
Use digital equipment such as data loggers for high-frequency sampling.
Maintain constant experimental conditions, especially temperature.
Stir solutions consistently to avoid concentration gradients.
Accurate measurement and safe working practices are essential skills for A-Level Chemistry, supporting reliable experiments and confidence when handling more advanced techniques later in the course.
FAQ
Choosing a salt bridge solution that does not react with either half-cell is essential. Potassium nitrate is common because its ions have similar mobilities and avoid junction potentials.
If the ions migrate at very different rates, the measured potential can drift over time.
A suitable salt bridge should:
Avoid forming precipitates with half-cell ions
Maintain constant ionic strength
Provide stable conduction without altering concentrations in each half-cell
Electrode surfaces naturally build up oxides, grease and impurities that hinder electron transfer. Cleaning removes these deposits, ensuring the electrode behaves according to its known standard potential.
Dirty electrodes may cause:
Lower or inconsistent EMF readings
Slow establishment of equilibrium
Poor reproducibility between trials
Common cleaning methods include gentle abrasion, acid rinsing or solvent wiping, depending on the metal involved.
Fluctuations often arise from incomplete equilibrium at the electrode surface or poor electrical contacts. Time is needed for ion concentrations near the electrode to stabilise.
Other causes include:
Drying or partial blockage of the salt bridge
Temperature changes
Contamination of solutions
Loose crocodile-clip connections
Ensuring stable conditions minimises drift and improves repeatability.
Temperature affects both the rate of reaction and the solubility or behaviour of gases measured during monitoring. Even slight variations can alter collision frequency and change measured rates.
To maintain consistency:
Use a thermostated water bath
Allow reactants to reach thermal equilibrium before starting
Avoid draughts or heat sources near the apparatus
Stable temperature ensures that observed rate changes arise from the reaction itself, not external influences.
Reliable colorimetry requires consistent light conditions and carefully prepared solutions. A clean, matched pair of cuvettes minimises reflection and scattering issues.
Improvements include:
Calibrating with a blank solution before each run
Using the wavelength corresponding to maximum absorbance
Ensuring thorough mixing to prevent concentration gradients
Avoiding bubbles or fingerprints on the cuvette surface
These steps help produce smooth absorbance–time curves for more accurate rate analysis.
Practice Questions
A student sets up an electrochemical cell using a zinc electrode in a solution of Zn2+ ions and a copper electrode in a solution of Cu2+ ions.
(a) State the function of the salt bridge. (1)
(b) Explain why a high-resistance voltmeter is used when measuring the cell potential. (2)
(3 marks)
(a) Salt bridge function (1 mark)
Allows movement of ions to complete the circuit / maintain electrical neutrality.
(b) High-resistance voltmeter (2 marks)
Prevents significant current from flowing. (1)
Ensures the measured potential difference is close to the true EMF of the cell. (1)
A reaction between magnesium and excess hydrochloric acid is monitored by measuring the volume of hydrogen gas produced every 15 seconds.
(a) State why this method is considered a continuous monitoring method. (1)
(b) Describe how the collected data can be used to determine the initial rate of reaction. (3)
(c) Suggest two improvements that would increase the accuracy of the rate measurements. (2)
(6 marks)
(a) Continuous method definition (1 mark)
Data are collected throughout the reaction at regular time intervals.
(b) Determining initial rate (3 marks)
Plot volume of hydrogen gas against time. (1)
Draw a tangent at t = 0. (1)
Calculate the gradient of the tangent to obtain the initial rate. (1)
(c) Improvements to accuracy (any two, 2 marks total)**
Use a gas syringe instead of collection over water for more precise volume measurements. (1)
Maintain a constant temperature throughout the experiment. (1)
Ensure airtight apparatus to prevent gas loss. (1)
Use digital data logging for more frequent and accurate readings. (1)

