OCR Specification focus:
'Use appropriate apparatus to record mass, time, volumes and temperature; use water baths, electric heaters or sand baths for heating safely.'
Introduction
Accurate measurement and controlled heating are fundamental to reliable results in A-Level Chemistry practical work. Mastering precision techniques ensures validity, repeatability, and safe experimental practice.
Core Measurement Techniques
Measuring Mass
Accurate mass measurement is vital for determining reagent quantities and analysing reaction yields. Electronic balances are commonly used, with varying degrees of precision depending on the required accuracy.
Analytical Balance: A precision instrument capable of measuring mass to 0.001 g or finer.
Before measuring, always ensure the balance is levelled, calibrated, and clean. Use weighing boats or watch glasses to hold substances, avoiding contamination and residue.
Key points when recording mass:
Zero or ‘tare’ the balance before adding the sample.
Avoid hot or wet samples, as these can cause mass instability.
Record all digits shown on the display to reflect the instrument’s uncertainty.
Close the draft shield and wait for the reading to stabilise before recording mass to the stated precision.

A modern analytical balance with an enclosed draft shield to minimise air currents and temperature fluctuations. Use clean containers (never place chemicals directly on the pan). Record mass only once the stable indicator is displayed. Source
Measuring Time
Time measurement underpins rate experiments and heating durations. Digital stopwatches or timers are standard, providing accuracy to 0.01 s.
Best practices:
Begin timing from a clear start signal (e.g., addition of reactant).
Stop timing precisely when a visible or measurable change occurs.
Repeat timings to obtain averages and improve reliability.
Measuring Volume
Accurate liquid measurement is central to quantitative chemistry, particularly in titrations and solution preparation.
Meniscus: The curved surface of a liquid caused by surface tension; readings are taken at the bottom of the meniscus at eye level for accuracy.
Apparatus used:
Measuring cylinders: Moderate accuracy, used for approximate volumes.
Pipettes (volumetric pipettes): Deliver a single fixed volume with high accuracy.
Burettes: Allow controlled delivery of varying volumes during titrations.
Volumetric flasks: Used for precise preparation of standard solutions.
Procedure tips:
Rinse apparatus with the solution being measured to prevent dilution errors.
Use a pipette filler to draw liquids safely.
Avoid parallax errors by aligning the eye with the meniscus.
Always read the bottom of the meniscus at eye level to avoid parallax error.
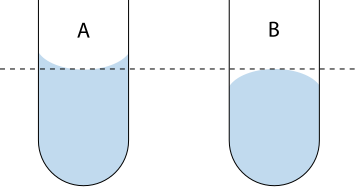
A clear diagram showing correct meniscus reading at eye level. For liquids like water that form a concave meniscus, the reading is taken at the lowest point. For liquids with a convex meniscus (e.g., mercury), read at the highest point. Source
Measuring Temperature
Temperature affects reaction rates and equilibria, so consistent measurement is crucial. Common instruments include liquid-in-glass thermometers, digital thermometers, and temperature probes connected to data loggers.
Data Logger: An electronic device that records measurements automatically over time for analysis.
Best practices:
Place the thermometer bulb fully immersed in the liquid, without touching the container.
Wait for readings to stabilise before recording.
Record temperature to the nearest 0.1 °C if possible.
When heating, ensure gradual temperature change to avoid overshooting the target.
Heating Methods
Water Baths
Water baths provide gentle, uniform heating, ideal for maintaining stable temperatures below 100 °C. They are frequently used in enzyme or reaction rate studies where precise temperature control is needed.
Advantages:
Reduces the risk of overheating or ignition.
Provides even heat distribution.
Suitable for substances sensitive to direct flame.
Always ensure the water level is above the liquid in the test tube but below the rim to avoid contamination.
Use water baths on a hotplate for temperatures up to around 100 °C; for higher temperatures use a sand bath (or an electric heater) as appropriate.
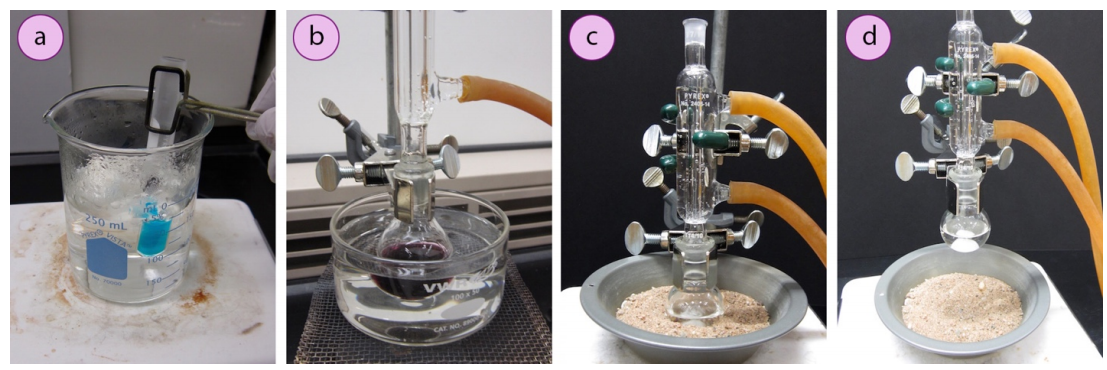
Photographs of a boiling water bath and sand bath used for controlled heating of flasks on a hotplate. Water baths are suitable up to ~100 °C, while sand baths accommodate higher temperatures. This page also shows an oil bath (extra detail beyond the OCR requirement but useful for differentiation). Source
Electric Heaters
Electric heating mantles and hotplates are common alternatives to open flames. They offer adjustable heat and reduce the risk of ignition when using flammable solvents.
Safety notes:
Never leave heating equipment unattended.
Check electrical cables and plugs for damage before use.
Use heat-resistant mats to protect the bench surface.
Sand Baths
A sand bath allows heating to higher and more controlled temperatures than a water bath, especially for volatile or flammable liquids. A beaker or dish filled with dry sand is placed on a hotplate, and the vessel containing the sample is nestled within the sand.
Advantages:
Provides gradual and uniform heating.
Reduces the risk of breakage due to thermal stress.
Safer for flammable or low-boiling substances compared to direct flame.
Safe Heating Practices
Key Safety Principles
Chemistry experiments often involve substances with various hazards. Always conduct a risk assessment before heating to identify and mitigate potential dangers.
Hazards and control measures:
Flammable substances: Use electric heaters or water baths, never open flames.
Toxic vapours: Carry out heating in a fume cupboard.
Corrosive materials: Wear goggles and gloves; handle glassware carefully.
Thermal burns: Use tongs or heatproof gloves when handling hot apparatus.
Monitoring Temperature and Avoiding Overheating
When heating, gradual temperature increase prevents bumping — sudden, violent boiling due to superheating of liquid. Using anti-bumping granules or continuous stirring distributes heat evenly and releases trapped vapour bubbles.
Bumping: Sudden, vigorous boiling caused by superheating in the absence of nucleation sites.
If heating under reflux, ensure apparatus connections are tight and the condenser is properly cooled with circulating water. Never seal a system completely, as pressure buildup may cause glassware to explode.
Recording and Reporting Measurements
Data Accuracy and Precision
Every measurement must include units and uncertainties. Consistency ensures results are comparable and scientifically valid.
Uncertainty: The range within which the true value of a measurement lies, reflecting instrument precision and human error.
When recording data:
Always note the instrument used.
Record to the correct number of significant figures based on apparatus sensitivity.
Repeat measurements and calculate means where appropriate.
Common Sources of Error
Instrumental errors: Miscalibrated apparatus or parallax error.
Environmental factors: Fluctuating temperature, pressure, or humidity.
Human error: Misreading scales or recording inconsistently.
Minimise these by careful calibration, repeating experiments, and consistent technique.
Integration with Digital Tools
Modern laboratories use data loggers and computer software for continuous measurement and recording. These tools improve accuracy, reduce human error, and facilitate easy data analysis and graphing.
Digital records also enhance traceability and allow for advanced error analysis, supporting scientific reliability in OCR practical investigations.
FAQ
Repeating measurements helps identify random errors and improve the reliability of results. By calculating the mean of repeated values, small inconsistencies caused by timing, reading instruments, or fluctuations in conditions can be minimised.
It also allows anomalies to be detected and excluded from analysis, providing a more accurate representation of true experimental values.
Residues from previous experiments can contaminate new samples and alter concentrations. Even small amounts of moisture can dilute solutions and introduce significant errors in volumetric analysis.
Always rinse measuring equipment with the solution to be used before starting to ensure accuracy and prevent dilution effects.
Uncertainty can be estimated from the instrument’s smallest scale division or manufacturer’s precision. For example:
A balance readable to 0.001 g has an uncertainty of ±0.001 g.
A thermometer with 1 °C divisions has an uncertainty of ±0.5 °C.
When multiple readings are taken, the mean can be used to reduce random uncertainty.
Several factors can influence accuracy:
The thermometer not being fully immersed in the liquid.
Reading the temperature before it stabilises.
Heat loss to the surroundings.
To improve accuracy, ensure good thermal contact between the sensor and liquid, use insulation where possible, and record data when equilibrium is reached.
Calibration ensures that an instrument provides correct readings within its expected range. Instruments can drift over time due to wear, contamination, or temperature changes.
Regular calibration against a standard reference guarantees accurate, traceable measurements and maintains consistency across experiments.
Practice Questions
A student is instructed to measure 25.0 cm³ of hydrochloric acid solution using a volumetric pipette. Explain how the student should ensure the reading of the liquid level in the pipette is accurate. (2 marks)
1 mark for stating that the reading should be taken at eye level.
1 mark for stating that the reading should be taken from the bottom of the meniscus.
A student heats an organic liquid using a water bath and later measures its temperature using a digital thermometer connected to a data logger.
(a) Explain why a water bath is used instead of heating the flask directly with a Bunsen burner. (2 marks)
(b) Suggest two ways the student can ensure accurate temperature readings. (2 marks)
(c) The student forgets to record the type of thermometer used in their report. Explain why this affects the reliability of the data. (1 mark)
(5 marks)
(a)
1 mark: Water bath provides gentle, even heating.
1 mark: Reduces risk of ignition or overheating of flammable substances.
(b)
1 mark: Ensure thermometer bulb is fully immersed in the liquid, not touching the container.
1 mark: Wait for the reading to stabilise or record using a data logger for consistent results.
(c)
1 mark: Different thermometers have different accuracies and uncertainties, affecting data reliability and comparison.

