OCR Specification focus:
'Set up distillation and heating under reflux; perform filtration, including fluted paper or reduced pressure; purify solids by recrystallisation or liquids using a separating funnel.'
Introduction
Separation and purification techniques are essential for isolating desired chemical products from reaction mixtures. These processes ensure chemical purity and accuracy in subsequent experimental analysis.
Distillation
Distillation separates components of a liquid mixture based on differences in boiling points. It is commonly used to purify liquids or collect volatile products from reactions.
Simple Distillation
Used when separating a liquid from a solution where boiling points differ by more than 25°C.
Process:
The mixture is heated in a round-bottom flask.
The liquid with the lower boiling point vaporises first.
Vapour travels through a condenser and is cooled back into liquid form.
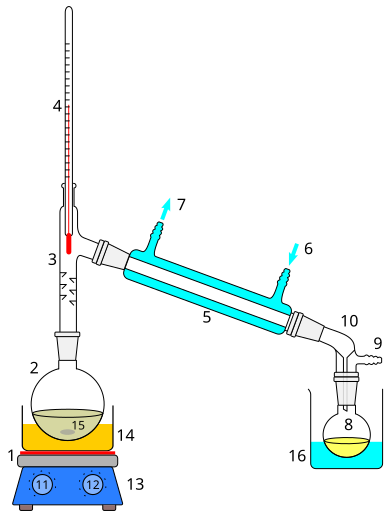
Labelled simple-distillation setup with heat source, distillation flask, still head, thermometer position, Liebig condenser, and receiving flask. The flow of cooling water is indicated to reinforce correct connection. This aligns with using distillation to purify a volatile liquid. Source
The purified distillate is collected in a receiver.
Fractional Distillation
Applied when components have similar boiling points.
Key apparatus: Fractionating column with glass beads or plates that provide multiple condensation and vaporisation stages, increasing separation efficiency.
Applications:
Separation of organic liquids with close boiling points.
Refining of crude organic products such as alcohols or esters.
Heating Under Reflux
Reflux allows a reaction to occur at its boiling temperature without losing volatile reactants or products.
Process:
The reaction mixture is heated in a round-bottom flask fitted with a vertical condenser.
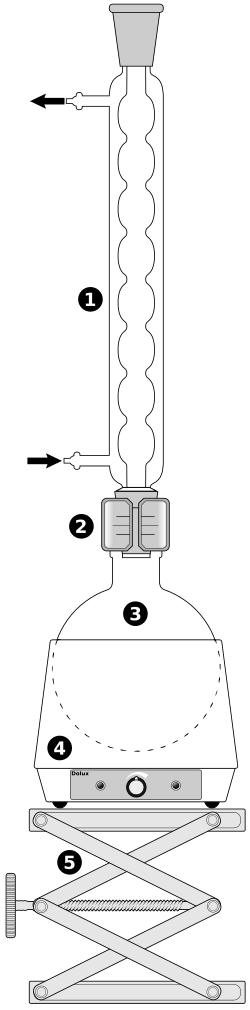
Labelled reflux apparatus showing an Allihn condenser mounted vertically above a round-bottom flask on a heating mantle. Condensed vapour returns to the flask, enabling prolonged heating without solvent loss. Water in/out ports are indicated to emphasise correct condenser orientation. Source
Vapours condense and drip back into the reaction flask, maintaining a constant volume and temperature.
The process ensures complete reaction by providing continuous heating.
Reflux: A technique used to heat a chemical reaction mixture for an extended period while preventing the loss of volatile substances through evaporation.
Safety note: The condenser must not be sealed to avoid pressure build-up, which could cause an explosion.
Filtration
Filtration separates solid impurities from liquid mixtures. Different filtration methods are selected depending on the particle size and purity required.
Gravity Filtration
Used when the filtrate (liquid) is the desired product.
Process:
The mixture is poured through a fluted filter paper in a funnel.
Gravity pulls the liquid through, leaving the solid residue behind.
Fluted filter paper increases surface area, improving filtration speed and efficiency.
Vacuum (Reduced Pressure) Filtration
Used when the solid product is desired, typically after recrystallisation.
Process:
A Büchner funnel and flask are connected to a vacuum pump.
Suction draws the liquid through the filter quickly.
The solid is left behind and can be washed and dried.
Vacuum Filtration: A method that uses suction to separate a solid from a liquid more efficiently than gravity filtration.
Safety considerations:
Ensure all glassware is clamped securely.
Avoid using vacuum filtration for volatile solvents, as rapid evaporation can cause implosion.
Recrystallisation
Recrystallisation purifies solid organic compounds by dissolving impurities and reforming crystals of the desired compound.
Process:
Dissolve the impure solid in the minimum amount of hot solvent.
Filter the hot solution to remove insoluble impurities.
Allow the solution to cool slowly, forming pure crystals.
Collect crystals by filtration and wash with a small volume of cold solvent.
Dry crystals thoroughly.
Recrystallisation: A purification process for solid compounds based on differences in solubility between the compound and its impurities in a chosen solvent.
Choosing the solvent:
The compound must be soluble when hot and insoluble when cold.
Impurities should either remain dissolved or be easily filtered out.
Common solvents: Water, ethanol, or acetone, depending on the solubility characteristics of the compound.
Separation of Liquids Using a Separating Funnel
A separating funnel isolates immiscible liquids (liquids that do not mix, e.g., organic and aqueous layers).
Process:
Pour the reaction mixture into the separating funnel.
Allow layers to form — the denser aqueous layer settles at the bottom, while the less dense organic layer floats above.

Separatory funnel mounted on a ring stand showing stopcock, stopper, and collection flask. The setup illustrates layering of immiscible phases and safe venting via the stopcock. Extra detail: the page also shows a separate image of venting, which, while not required by the syllabus, reinforces safe technique. Source
Remove the lower layer by opening the tap carefully.
Wash the remaining layer with water or other solvents to remove impurities.
Dry the purified liquid using a drying agent such as anhydrous magnesium sulfate.
Immiscible Liquids: Liquids that do not mix to form a homogeneous solution, instead forming separate layers based on density.
Safety notes:
Vent the funnel frequently to release pressure caused by volatile solvent vapours.
Ensure correct identification of layers before collection.
Common Reaction Setups in Separation and Purification
1. Reflux Setup
Apparatus: Round-bottom flask, vertical condenser, heat source, anti-bumping granules.
Purpose: Maintain consistent heating for reactions involving volatile reactants.
2. Distillation Setup
Apparatus: Round-bottom flask, condenser, thermometer, receiving flask.
Purpose: Purify or collect volatile liquids by exploiting boiling point differences.
3. Filtration and Recrystallisation Setup
Apparatus: Büchner funnel, Büchner flask, vacuum pump, hot plate.
Purpose: Purify solid products and remove impurities efficiently.
4. Separating Funnel Setup
Apparatus: Separating funnel, retort stand, beaker, drying agent.
Purpose: Separate and purify immiscible liquid layers.
Practical Considerations and Safety
Hazards and Risk Minimisation:
Heat sources: Use water baths, electric heaters, or sand baths to prevent open flames near flammable solvents.
Chemical handling: Always wear lab coats, gloves, and goggles when using corrosive or irritant substances.
Waste disposal: Organic and aqueous wastes must be disposed of separately according to laboratory protocols.
Best practices:
Always label glassware and use clamps to prevent accidents.
Check for cracks in glass equipment before applying vacuum or heat.
Record all observations and temperatures accurately for reproducibility.
FAQ
Simple distillation is used when separating a liquid from a solution or from another liquid with a boiling point difference greater than 25°C. It involves a single vaporisation-condensation cycle.
Fractional distillation, however, is suitable when the boiling points of the components are close together. A fractionating column provides multiple vaporisation-condensation stages, improving separation efficiency.
In summary:
Simple distillation: Large boiling point difference.
Fractional distillation: Small boiling point difference.
Heating under reflux ensures that volatile reactants and products remain in the reaction mixture, allowing the reaction to proceed to completion without loss of material.
This is particularly crucial for reactions requiring high temperatures or long durations, as the condenser continuously cools and returns vapours to the flask.
Without reflux, volatile compounds could evaporate, resulting in incomplete reactions and poor yields.
Recrystallisation relies on differences in solubility between the desired compound and its impurities.
The process involves dissolving the crude product in a hot solvent and allowing it to cool slowly. The pure compound crystallises out, while impurities remain dissolved or are removed during filtration.
To achieve maximum purity:
Use the minimum amount of hot solvent.
Allow slow cooling to form well-defined crystals.
Wash the final crystals with cold solvent to remove traces of impurities.
A suitable solvent must dissolve the substance when hot but not when cold. The solubility profile determines how efficiently impurities can be separated.
Key considerations:
The desired compound should crystallise out on cooling.
Impurities should either stay dissolved or be easily filtered out.
The solvent must not chemically react with the compound.
Common examples include water, ethanol, or acetone, depending on the polarity of the compound.
Several procedural errors can lead to loss of product or unsafe conditions:
Not venting regularly: Pressure build-up from volatile solvents can cause the stopper to pop off.
Incorrect layer identification: Misjudging which layer is aqueous or organic may result in discarding the product.
Incomplete separation: Failing to allow sufficient time for layers to settle can lead to contamination.
Good practice includes labelling collection containers, venting frequently, and confirming layer identity using small-scale tests.
Practice Questions
Explain why a condenser is used during heating under reflux in an organic synthesis reaction. (2 marks)
1 mark: States that the condenser prevents loss of volatile reactants or products.
1 mark: Mentions that vapours condense and return to the reaction flask, allowing continuous heating without evaporation losses.
A student prepares an organic product that must be purified after the reaction. Describe how the student could purify and separate the product using suitable laboratory techniques, including any safety precautions that should be taken. (5 marks)
1 mark: Mentions use of a separating funnel to separate immiscible layers (organic and aqueous).
1 mark: States that the organic layer should be washed with water or other solvents to remove impurities.
1 mark: Describes drying the organic layer using a drying agent such as anhydrous magnesium sulfate.
1 mark: Mentions distillation (simple or fractional) to purify the liquid based on boiling point differences.
1 mark: Includes at least one safety precaution, e.g., venting the funnel regularly, using a water bath for heating, or wearing goggles and gloves.

