OCR Specification focus:
‘Use the terms empirical formula and molecular formula, and calculate both from mass or percentage composition and relative molecular mass.’
Introduction
Understanding empirical and molecular formulae is essential for analysing chemical composition. This subtopic focuses on defining these formula types and determining them using composition data and relative molecular mass.
Empirical and Molecular Formulae: Core Concepts
An empirical formula represents the simplest whole-number ratio of atoms of each element in a compound. The molecular formula shows the actual number of atoms of each element in a molecule.
Empirical formula: The simplest whole-number ratio of atoms of each element in a compound.
Empirical formulae help chemists describe composition even when the exact molecular structure is unknown.
Molecular formula: The actual number of atoms of each element present in a molecule of the compound.
These two formulae are closely linked. A molecular formula is always an integer multiple of its empirical formula, and this relationship becomes central when interpreting composition data.
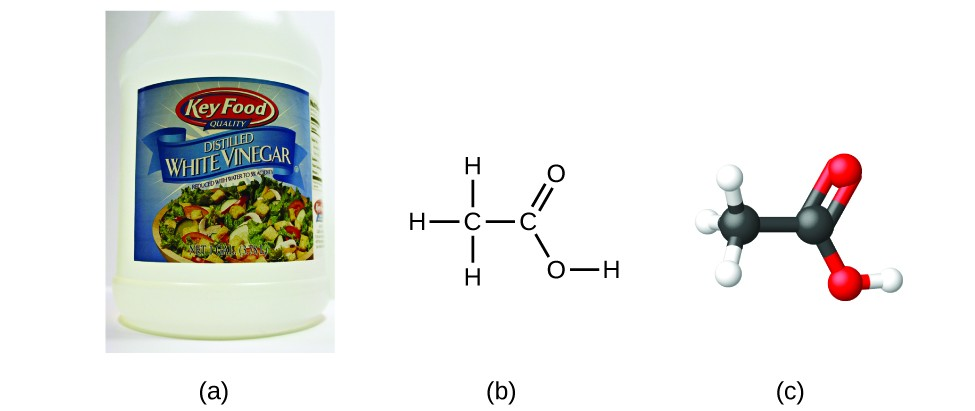
Acetic acid in vinegar has molecular formula C₂H₄O₂ but empirical formula CH₂O, illustrating the whole‑number multiple relationship between molecular and empirical formulae. The image shows a bottle of white vinegar, its line structural formula, and a ball‑and‑stick model. The vinegar photograph goes beyond OCR’s scope but supports conceptual understanding. Source
Using Composition Data to Determine Formulae
To meet OCR expectations, students must be able to use mass or percentage composition to determine empirical formulae, then use relative molecular mass to identify molecular formulae. This process relies on converting masses into moles and analysing ratios.
Converting Mass or Percentage to Empirical Formula
The determination of an empirical formula always involves identifying the simplest ratio of amounts of each element. Percentage composition can be treated as mass in grams for this purpose.
The fundamental approach includes:
Recording masses or percentage composition of each element.
Converting each mass into moles using molar masses from the Periodic Table.
Determining the simplest whole-number ratio by dividing all molar amounts by the smallest value.
Scaling the ratio when necessary so that all numbers are whole numbers.
These steps ensure alignment with OCR's requirement to calculate empirical formulae accurately from composition data.
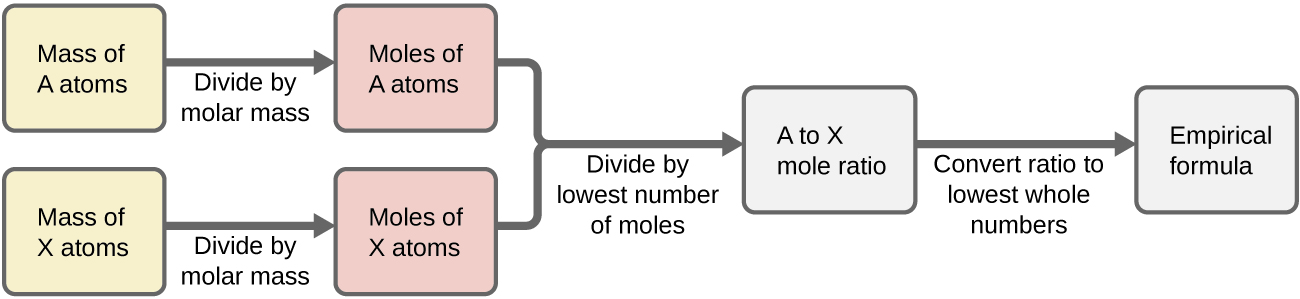
Flowchart showing the steps for determining an empirical formula from elemental masses. Masses are converted to moles, ratios simplified, and the empirical formula obtained. The layout closely models the OCR‑required method for empirical formula determination. Source
Importance of Whole-Number Ratios
Empirical formula calculations must always result in whole numbers. When division produces decimals close to whole numbers (e.g. 1.99 or 1.33), scaling is required. Organic compounds commonly produce ratios that need multiplying, reinforcing the need for precision in ratio evaluation.
From Empirical Formula to Molecular Formula
Once the empirical formula is known, the molecular formula can be found using the relative molecular mass (Mr). OCR requires accurate use of Mr to scale the empirical formula up to match the actual molecular mass.
Relative molecular mass (Mr): The weighted mean mass of a molecule compared with one-twelfth of the mass of an atom of carbon-12.
The link between empirical and molecular formulae depends on identifying how many empirical units fit into the molecule. This is done using a ratio:
Molecular Formula Scaling Factor (n) = Mr of compound ÷ Mr of empirical formula
n = Number of empirical units in the molecule (no units)
Once n is known, each subscript in the empirical formula is multiplied by this factor to give the molecular formula.
Why Relative Molecular Mass Is Essential
Relative molecular mass is necessary because some compounds share the same empirical formula but have different molecular structures. Without Mr, it would be impossible to determine the true molecular formula from composition alone. For example, distinct compounds may share a simple ratio such as CH, but vary widely in molecular size. The Mr value therefore ensures accurate identification of the actual molecular composition.
Sources of Relative Molecular Mass Data
Students will obtain Mr values from:
Mass spectrometry data (covered in other subsubtopics).
Given information in exam questions.
Established chemical data sources, such as Periodic Table atomic masses.
This ensures the scaling process is grounded in precise numerical data.
Structural Implications of Formulae
Although structural chemistry belongs to different syllabus areas, empirical and molecular formulae provide foundation information about composition. While the empirical formula offers only elemental ratios, the molecular formula gives additional insight into:
Possible structural isomers,
The degree of saturation,
The general class of compound.
However, determining structure requires further analytical information and is not part of this subsubtopic.
Common Pitfalls When Determining Formulae
OCR expects students to recognise and avoid typical errors when performing formula calculations. Key issues include:
Misinterpreting Percentage Composition
Students sometimes forget that percentage composition is treated as mass in grams. To avoid this:
Always assume 100 g of compound, meaning percentages translate directly into masses.
Failing to Obtain Whole Numbers
If ratios such as 1.5 or 2.33 occur:
Multiply all values by the smallest factor that removes decimals (e.g. ×2 or ×3).
Confusing Empirical and Molecular Formulae
Remember:
Empirical = simplest ratio
Molecular = actual numbers of atoms
Molecular formula must be an integer multiple of empirical formula.
These distinctions must be applied consistently.
Checklist of Skills Required by OCR
Students should ensure they can:
Use mass or percentage composition to calculate empirical formulae.
Use relative molecular mass to calculate molecular formulae.
Convert between empirical and molecular forms confidently.
Interpret formula data without structural assumptions.
Present formulae clearly and in correct chemical notation.
Applying These Skills in Context
Mastering empirical and molecular formulae supports deeper chemical understanding across many topics that follow, including organic chemistry, stoichiometry, and analytical interpretation. Accurate formula determination forms a basis for more advanced problem-solving involving composition, reaction pathways and the quantitative interpretation of chemical data.
FAQ
When ratios fall between expected whole numbers, chemists look for simple fractional patterns such as halves, thirds, or quarters.
If values like 1.50, 1.33 or 1.25 appear, they multiply all ratios by the smallest integer that removes the fraction.
1.50 suggests multiplying by 2
1.33 suggests multiplying by 3
1.25 suggests multiplying by 4
This avoids forcing an incorrect formula and ensures the final ratio reflects a true underlying molecular composition.
The empirical formula only shows the simplest element ratio, not the structure or bonding arrangement.
Compounds with identical empirical formulae may differ in:
molecular size
functional groups
atom connectivity
intermolecular forces
These structural variations can produce entirely different physical and chemical properties despite identical empirical ratios.
Percentage composition is typically found using analytical techniques that determine element masses within a compound.
Common methods include:
combustion analysis for carbon and hydrogen
nitrogen determination using Dumas or Kjeldahl methods
instrumental analysis such as mass spectrometry or elemental analysers
Each technique provides accurate elemental mass data used to calculate percentage composition prior to empirical formula determination.
Yes. Small measurement errors can significantly influence mole ratios, especially when elements are present in similar amounts.
Sources of error include:
inaccurate mass measurements
incomplete combustion or decomposition
contamination of samples
rounding too early in calculations
Careful experimental technique and carrying full decimal precision until the final step help avoid incorrect ratios.
The molecular formula sets limits on the possible structures a compound can have, which helps direct further analysis.
It enables chemists to:
predict possible functional groups
assess degrees of unsaturation
narrow down structural isomers
select suitable spectroscopic methods for confirmation
Although it does not show the structure itself, the molecular formula provides essential constraints for structural determination.
Practice Questions
A compound contains 27.3% carbon and 72.7% oxygen by mass.
Calculate the empirical formula of the compound. Show your reasoning.
(2 marks)
Convert percentages to moles:
C: 27.3 / 12 = 2.275; O: 72.7 / 16 = 4.544 (1 mark)
Divide by smallest to obtain ratio 1 : 2 → empirical formula CO2 (1 mark)
A compound used in industry contains carbon, hydrogen, and nitrogen.
A 0.500 g sample of the compound is found to contain:
0.210 g carbon
0.070 g hydrogen
0.220 g nitrogen
(a) Determine the empirical formula of the compound.
(b) The relative molecular mass (Mr) of the compound is 60.
Determine the molecular formula.
Show all steps clearly.
(5 marks)
(a) Empirical formula (3 marks)
Convert masses to moles:
C: 0.210 / 12 = 0.0175
H: 0.070 / 1 = 0.070
N: 0.220 / 14 = 0.0157 (1 mark)
Divide by smallest to obtain whole-number ratio:
C: 1.11, H: 4.46, N: 1.00 (1 mark)
Convert to whole numbers → empirical formula CH4N (1 mark)
(b) Molecular formula (2 marks)
Calculate Mr of empirical formula CH4N:
12 + 4(1) + 14 = 30 (1 mark)
Scaling factor = 60 / 30 = 2 → molecular formula C2H8N2 (1 mark)

