OCR Specification focus:
‘Use stoichiometric relationships; calculate percentage yield and atom economy; understand measurement techniques and sustainability benefits of high atom economy.’
Stoichiometry underpins quantitative chemistry by linking amounts of reactants and products using balanced equations. This section explores stoichiometric relationships, percentage yield, atom economy, and key measurement techniques.
Stoichiometric Relationships
Stoichiometry involves using balanced chemical equations to determine the quantitative relationships between species in a reaction. A balanced equation shows the mole ratios in which substances react and form products. These ratios allow predictions about the amount of product obtained or reactant required.
When applying stoichiometry, students must identify the molar ratio between relevant reactants and products, convert between mass, moles and volume where needed, and ensure equations are correctly balanced before performing any quantitative work. Stoichiometry is essential for interpreting reaction efficiency and sustainability metrics later in this topic.
Percentage Yield
Percentage yield provides a measure of how much product is actually obtained compared with the theoretical maximum amount predicted by stoichiometry. When a reaction does not proceed fully or side reactions occur, the yield falls below the theoretical value.
Percentage yield: The proportion of theoretical product mass actually obtained in a reaction, expressed as a percentage.
A sentence explaining practicability: Yield is influenced by incomplete reactions, competing processes, product loss during purification, and reversible reaction equilibria.
Percentage yield compares the actual mass of product obtained with the theoretical mass calculated from stoichiometry.
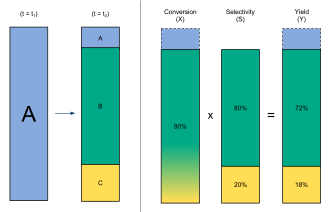
This diagram relates yield to other measures of reaction performance, including conversion and selectivity. It reinforces how yield compares actual product to theoretical predictions. The references to conversion and selectivity go beyond OCR requirements but help contextualise yield within efficiency metrics. Source
Percentage Yield (%) = (Actual Yield ÷ Theoretical Yield) × 100
Actual Yield = Mass of product collected (g)
Theoretical Yield = Maximum mass predicted by stoichiometry (g)
Understanding percentage yield allows chemists to improve reaction conditions, minimise waste, and evaluate industrial efficiency.
Atom Economy
Atom economy measures how efficiently atoms from reactants are incorporated into the desired product. It is a sustainability-focused metric, emphasised in modern green chemistry. A high atom economy indicates less waste produced and a more efficient process.
Atom economy: The proportion of the total mass of reactants that becomes the desired product, expressed as a percentage.
Unlike percentage yield, which depends on practical execution, atom economy reflects the inherent efficiency of the reaction equation.
High atom economy reactions minimise waste because most atoms in the reactants appear in the desired product.

This diagram illustrates how atoms from reactants are allocated between desired product and waste. A higher atom economy means more atoms are incorporated into useful products. It includes broader green-chemistry context beyond the OCR specification. Source
Atom Economy (%) = (Mr of Desired Product ÷ Total Mr of All Products) × 100
Mr = Relative formula mass of species
Atom economy is central to evaluating the environmental impact of chemical processes. Reactions producing multiple by-products typically have lower atom economies, whereas addition reactions often have atom economies close to 100%.
Techniques Relevant to Stoichiometry
The specification requires understanding of how practical techniques affect the reliability of measurements used in stoichiometric calculations. Accurate measurements underpin reliable yield and atom economy calculations.
Essential Measurement Techniques
Chemical reactions depend on precise measurement of masses, volumes and concentrations. Students should be confident in using laboratory equipment to obtain reproducible data.
Important measurement considerations include:
Ensuring balances are calibrated and masses recorded to appropriate decimal places.
Reading burette and pipette volumes at eye level, using the bottom of the meniscus.
Using volumetric flasks to prepare solutions of known concentration with high precision.
Considering temperature effects on gas volume measurements.
Minimising transfer losses during procedures.
Accurate volumetric glassware such as burettes, pipettes and volumetric flasks is essential for obtaining reliable yields in stoichiometric experiments.
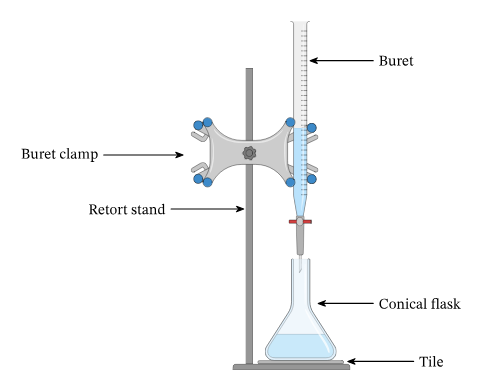
This diagram shows a standard titration arrangement used to obtain precise volume measurements. It highlights the role of burettes and conical flasks in generating accurate experimental data. Extra details about indicator use appear but remain helpful for understanding technique. Source
Techniques Contributing to Percentage Yield
Certain practical steps can reduce the yield unintentionally. These do not affect atom economy but do influence the actual mass collected.
Examples include:
Product adhesion to apparatus surfaces, such as beakers or filters.
Incomplete reactions, where not all reactants convert into product.
Side reactions, creating undesired by-products.
Purification losses, for instance during recrystallisation or filtration.
These factors highlight why percentage yield rarely reaches 100%.
Techniques Contributing to Accuracy in Stoichiometric Calculations
Stoichiometric relationships are only valid when experimental data are reliable. The following practices enhance accuracy:
Using volumetric glassware to measure solutions, not measuring cylinders.
Drying solids thoroughly before weighing to avoid absorbed moisture skewing results.
Rinsing apparatus with appropriate solutions to prevent dilution or contamination.
Performing multiple titrations to achieve concordant titre values.
Using closed systems where appropriate to prevent gaseous reactants or products escaping.
These techniques reduce uncertainty and ensure calculated amounts reflect the true course of the reaction.
Sustainability and the Importance of High Atom Economy
Atom economy is a key principle of green chemistry. Reactions with high atom economies minimise waste, conserve raw materials, and reduce the environmental impact of chemical manufacture. This aligns with industrial goals to improve process sustainability and comply with environmental regulations.
High atom economy is particularly valuable in large-scale synthesis, where even small improvements significantly reduce waste generation. Industries increasingly favour processes such as addition reactions or catalytic pathways that maximise incorporation of atoms into the principal product.
Furthermore, atom economy encourages chemists to design reactions that avoid hazardous by-products, making chemical processes safer for both operators and the environment. Yield remains important for practical efficiency, but atom economy reflects the broader sustainability value of the reaction pathway.
Integrating Stoichiometry, Yield and Atom Economy
Stoichiometric analysis provides the theoretical framework for calculating both percentage yield and atom economy. Together, these concepts allow chemists to assess reactions from both practical and environmental perspectives. Understanding experimental techniques ensures the data used in these calculations are robust, supporting informed decisions in laboratory and industrial settings.
FAQ
Percentage yield can be reduced by losses during product transfer, such as material left in beakers, on spatulas, or trapped in filter paper.
Impurities may also reduce the measured mass if the product retains solvent or moisture.
In reversible reactions, equilibrium constraints prevent complete conversion of reactants.
Atom economy helps companies evaluate long-term sustainability and cost efficiency. A route with a higher atom economy usually produces less waste, reducing disposal costs.
Industries may prioritise processes that:
Use fewer steps
Avoid hazardous by-products
Require fewer purification stages
This improves environmental compliance and resource efficiency.
A reaction with excellent atom economy may have slow reaction rates or require extreme temperatures or pressures.
It may also use expensive catalysts or reagents that make large-scale production uneconomical.
Purification demands or safety risks can also outweigh atom economy benefits.
Chemists ensure gas volumes are measured under controlled conditions.
They may:
Use gas syringes rather than displacement of water
Maintain constant temperature to prevent expansion or contraction
Check for leaks in delivery tubes
Use dry gases where moisture could interfere
These steps keep molar volume calculations reliable.
A suitable solvent ensures reactants dissolve properly, enabling complete mixing and reducing incomplete reactions.
Some solvents minimise side reactions or decomposition.
Poorly chosen solvents can retain product, lowering yield, or cause evaporation losses that affect mass measurements.
Practice Questions
A reaction has a theoretical yield of 12.0 g of product. A student obtains 9.0 g of product from the reaction.
Calculate the percentage yield of the reaction. Show your working. (2 marks)
Correct equation used: percentage yield = (actual yield / theoretical yield) × 100 (1 mark)
Percentage yield correctly calculated: (9.0 / 12.0) × 100 = 75% (1 mark)
A chemist is comparing two different synthetic routes to prepare the same organic product.
Route A produces the desired product and one by-product.
Route B is an addition reaction that forms only the desired product.
(a) Explain the term atom economy. (2 marks)
(b) State which route, A or B, has the higher atom economy and explain your reasoning. (2 marks)
(c) Give one sustainability benefit of using a route with a high atom economy. (1 mark)
(5 marks)
(a)
Atom economy measures the proportion of the mass of reactants that becomes the desired product (1 mark)
Expressed as a percentage or includes the idea of minimising waste (1 mark)
(b)
Route B has the higher atom economy (1 mark)
Addition reactions form only one product, so all atoms from reactants are incorporated into the desired product (1 mark)
(c)
One sustainability benefit correctly stated, e.g. less waste, better use of raw materials, reduced environmental impact, lower disposal costs (1 mark)

