OCR Specification focus:
‘Explain and use amount of substance, mole (mol), Avogadro constant NA, molar mass (g mol–1) and molar gas volume (dm³ mol–1).’
Understanding the mole, the Avogadro constant, and key quantitative terms is essential for mastering chemical calculations, enabling accurate interpretation of substance amounts in reactions and laboratory processes.
The Mole and Amount of Substance
The concept of amount of substance lies at the heart of quantitative chemistry and provides a consistent way to relate the number of particles to measurable quantities such as mass, gas volume, and concentration.
The Mole
Chemists use the mole as the standard unit for amount of substance because it links the microscopic world of atoms and molecules to the macroscopic quantities measured in the laboratory.
One mole of any substance contains 6.022 × 10²³ particles, a fixed number called the Avogadro constant, Nₐ.
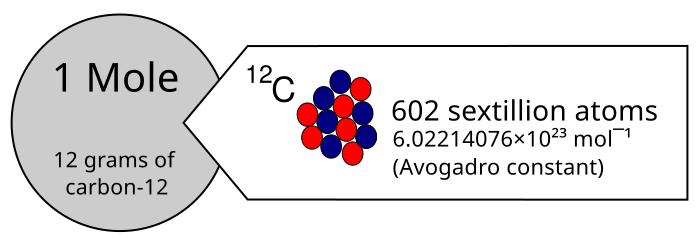
This diagram shows 1 mole of carbon‑12 as a 12 g sample containing 6.02214076 × 10²³ atoms. It links the unit ‘mole’ to particle number and mass. It reflects the historical carbon‑12 definition underpinning the modern fixed value of the Avogadro constant. Source
Mole (mol): The amount of substance containing exactly 6.022 × 10²³ particles, defined by the Avogadro constant.
A mole can refer to atoms, molecules, ions, electrons, or formula units, depending on the chemical species being considered. The value of the Avogadro constant is fixed and underpins all mole-based calculations encountered in A-Level Chemistry.
The Avogadro Constant
The Avogadro constant (NA) gives the number of particles in one mole of a substance and provides the essential link between mass and number of particles.
Avogadro Constant (NA): The number of particles in one mole, equal to 6.022 × 10²³ mol⁻¹.
This constant applies universally and is independent of the type of particle involved. It enables chemists to convert between mass quantities and actual numbers of atoms or molecules, a key skill required by the OCR specification.
Molar Mass and Its Use
The molar mass provides the mass of one mole of a substance and is expressed in g mol⁻¹. It is numerically equal to the relative atomic or relative molecular mass found on the periodic table.
Molar Mass in Chemical Calculations
Molar mass serves as the bridge between grams and moles, allowing chemists to compare substances based on particle numbers rather than physical mass.
Molar Mass (M): The mass of one mole of a substance, expressed in g mol⁻¹.
The concept of molar mass applies to elements, simple molecules, ions, and larger structures. OCR A-Level Chemistry frequently requires converting mass to moles and vice versa using molar mass relationships.
Molar Gas Volume
For gases, the mole concept extends to relationships involving gas volume, which can behave predictably under standard conditions.
Gas Volume per Mole
At standard conditions (commonly used in A-Level contexts), one mole of any gas occupies a defined molar gas volume, enabling simple conversions between moles and volumes.
Molar Gas Volume: The volume occupied by one mole of gas under specified conditions, typically 24 dm³ mol⁻¹ at room temperature and pressure.
Understanding molar gas volume allows students to compare different gases in terms of the number of particles rather than by their individual masses or densities.
Key Relationships in Amount of Substance
A number of essential quantitative relationships allow chemists to calculate masses, volumes, and particle numbers from the mole concept.
The Fundamental Mole Equation
One of the most important relationships connects amount of substance to mass and molar mass.
Mass–Mole Relationship (n = m/M)
n = amount of substance (mol)
m = mass of substance (g)
M = molar mass (g mol⁻¹)
This relationship lies at the core of virtually all stoichiometric calculations and supports predicting quantities in chemical reactions.
These relationships between mass, amount of substance in moles, number of particles and gas volume can be summarised in a single diagram with the mole at the centre.
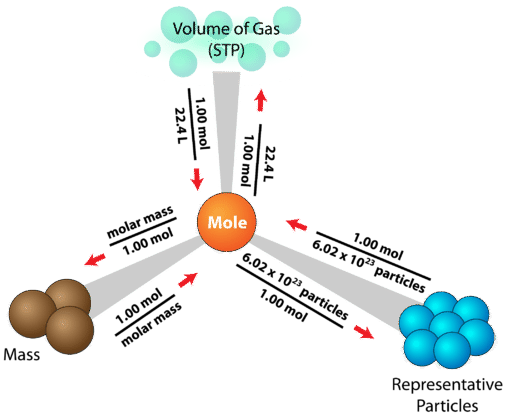
This diagram places the mole at the centre of a map linking mass, particles, and gas volume. Each arrow shows the conversion factor used in moving between these quantities. It uses 22.4 L at STP rather than the 24.0 dm³ mol⁻¹ at r.t.p. used in OCR Chemistry. Source
A separate but equally important relationship links the number of particles to the amount of substance.
Particles–Mole Relationship (n = N/NA)
n = amount of substance (mol)
N = number of particles
NA = Avogadro constant (mol⁻¹)
These equations provide the mathematical foundation for interpreting chemical equations quantitatively, aligning directly with OCR's requirement to “explain and use amount of substance, mole, Avogadro constant, molar mass and molar gas volume.”
Practical Uses of the Mole Concept
The mole underpins nearly all quantitative chemistry, and mastering the associated terminology helps students interpret laboratory data and theoretical chemical relationships effectively.
Why the Mole Is Essential
Key applications within the OCR A-Level specification include:
Determining reactant quantities for chemical reactions.
Interpreting balanced chemical equations, which represent mole ratios.
Converting between mass, gas volume, and particle number.
Expressing the composition of substances in terms of moles rather than mass.
Relating chemical formulas to measurable amounts using molar mass.
Common Terminology Students Must Recognise
A-Level Chemistry students are expected to confidently interpret the following key terms, all of which are essential components of this subsubtopic:
Amount of substance – a quantity measured in moles.
Mole (mol) – the base unit linking particles to macroscopic measurements.
Avogadro constant (NA) – numerical value connecting moles to numbers of particles.
Molar mass (g mol⁻¹) – mass of one mole of a substance.
Molar gas volume (dm³ mol⁻¹) – gas volume for a mole under specified conditions.
At room temperature and pressure (r.t.p.), one mole of any ideal gas has the same molar gas volume, taken in OCR Chemistry as about 24.0 dm³ mol⁻¹.
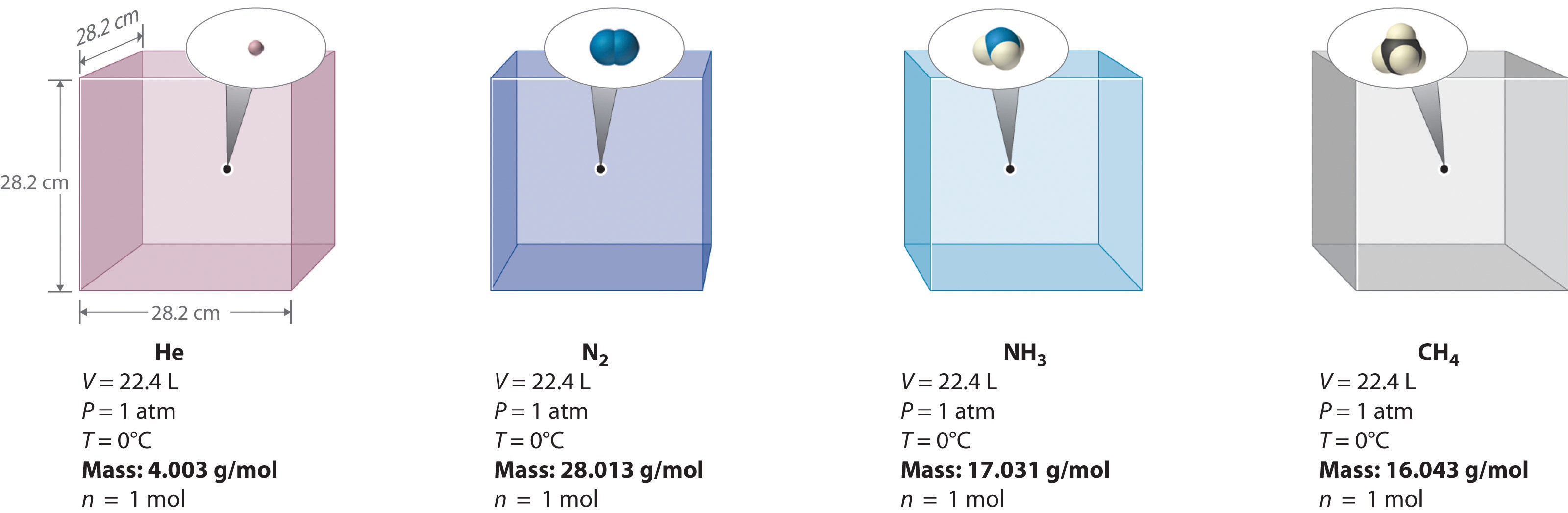
This image shows four gas samples at STP, each 22.4 L and containing 1 mol of gas. It illustrates that equal gas volumes at identical conditions contain the same number of particles. It uses STP rather than r.t.p., but the mole relationship remains the same. Source
Developing Fluency with These Concepts
A-level students must be able to apply these definitions and equations fluently in varied contexts, including mass calculations, gas volume conversions, and interpreting experimental data. These foundational concepts reappear throughout the OCR A-Level Chemistry course and form the basis of quantitative chemical reasoning.
FAQ
The modern definition fixes the Avogadro constant at exactly 6.02214076 × 10^23 mol–1, meaning the mole is defined by the number of particles rather than the mass of carbon-12.
Historically, one mole was defined as the amount of substance containing the same number of atoms as exactly 12 g of carbon-12. The modern definition removes dependence on a measured mass and increases precision in measurements.
Molar gas volume depends on the temperature and pressure conditions chosen. Different boards adopt slightly different “standard” conditions.
OCR typically assumes room temperature and pressure (about 24 dm3 mol–1), whereas older texts use standard temperature and pressure (22.4 dm3 mol–1).
These values are approximations for ideal gases and may vary with updated scientific conventions.
Yes. The molar mass of an element depends on the relative abundance of its isotopes.
Elements with significant variation in isotopic ratios (such as chlorine or copper) may have molar masses that differ slightly in natural samples.
In calculations, the periodic table value reflects the weighted average molar mass used for standard conditions.
Atoms and molecules exist in quantities far too large to count directly, making the mole a convenient scaling unit.
Using moles allows chemists to relate measurable laboratory quantities such as mass, volume, and concentration to particle numbers.
It also provides a consistent basis for interpreting balanced chemical equations, which represent mole ratios.
Gas volume increases with temperature due to greater kinetic energy and particle movement.
For a fixed amount of gas at constant pressure:
Higher temperature increases volume
Lower temperature decreases volume
This is why molar gas volume is always quoted with reference to a specific set of conditions, such as r.t.p. for OCR Chemistry.
Practice Questions
Define the term mole and state the value of the Avogadro constant.
(2 marks)
Correct definition of mole: the amount of substance containing 6.022 × 10^23 particles (1 mark)
Correct value of the Avogadro constant: 6.022 × 10^23 mol–1 (1 mark)
A sample of a gaseous element has a mass of 9.6 g. The molar mass of the element is 32 g mol–1.
(a) Calculate the amount of substance, in moles, in the sample. (2 marks)
(b) State the number of particles present in this amount of substance. (1 mark)
(c) Explain why equal volumes of different gases at the same temperature and pressure contain the same number of particles. (2 marks)
(5 marks)
(a)
Uses n = m / M or equivalent method shown (1 mark)
Correct answer: 0.30 mol (allow 0.3 mol) (1 mark)
(b)
Number of particles = 0.30 × 6.022 × 10^23 = 1.81 × 10^23 (allow appropriate rounding) (1 mark)
(c)
States that gas particles are far apart and have negligible volume (ideal gas behaviour) (1 mark)
Therefore, at the same temperature and pressure, gases contain the same number of particles per unit volume (1 mark)

