OCR Specification focus:
'Define an atomic orbital and recognise the shapes of s and p orbitals; each orbital holds up to two electrons with opposite spins.'
Introduction
Atomic orbitals describe regions around an atom’s nucleus where electrons are most likely found. Understanding their shapes and capacities is key to predicting chemical behaviour.
Understanding Atomic Orbitals
Definition of an Atomic Orbital
Atomic Orbital: A region around an atom’s nucleus where there is a high probability of finding an electron.
Electrons do not orbit the nucleus in fixed paths like planets; instead, they occupy these probabilistic regions. The concept of orbitals arises from quantum mechanics, particularly from solving the Schrödinger equation for electrons in atoms. Each orbital has a specific energy and shape, defined by quantum numbers.
Quantum Numbers and Orbital Description
Each electron in an atom is described by a unique set of quantum numbers, which define its energy, shape, orientation, and spin:
Principal quantum number (n): Indicates the main energy level or shell.
Angular momentum quantum number (l): Determines the shape of the orbital.
Magnetic quantum number (ml): Describes the orientation of the orbital in space.
Spin quantum number (ms): Describes the direction of electron spin, either +½ or –½.
These quantum numbers together define an electron’s precise quantum state within the atom.
The Shape and Nature of Orbitals
The s Orbital
The s orbital corresponds to an angular momentum quantum number (l = 0). It has a spherical shape centred around the nucleus.
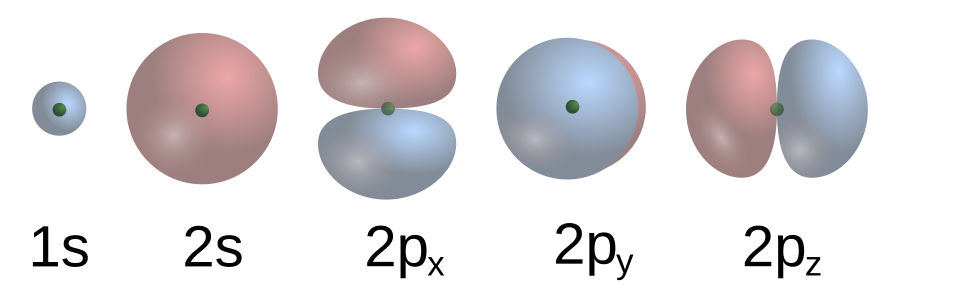
s and p orbital shapes. The s orbital is spherical and non-directional, whereas p orbitals are dumbbell-shaped and oriented along perpendicular axes. This diagram focuses on visual recognition required by the specification. Source
Each energy level contains one s orbital: 1s, 2s, 3s, etc.
The size of the s orbital increases with increasing principal quantum number (n).
For example, a 2s orbital is larger and higher in energy than a 1s orbital.
The electron density is greatest near the nucleus and gradually decreases with distance. This spherical symmetry means that an s orbital has no directional preference, making it crucial for forming sigma bonds in covalent compounds.
The p Orbitals
When the angular momentum quantum number l = 1, the orbitals are p orbitals. These are dumbbell-shaped and oriented along the x, y, and z axes.
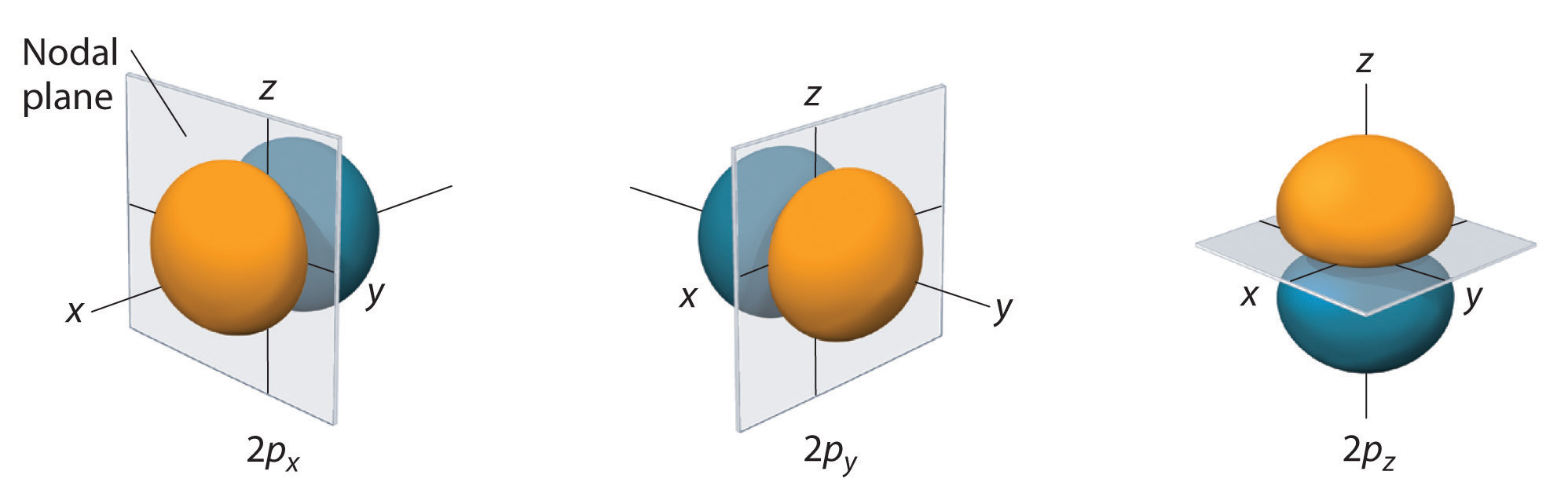
The three 2p orbitals (px, py, pz) and their nodal planes. Each p orbital is oriented along one Cartesian axis and contains a planar node through the nucleus. Colouring indicates wavefunction phase, which is extra detail not required by the syllabus but does not affect shape recognition. Source
There are three p orbitals in each p sub-shell: px, py, and pz.
The shapes are identical but differ in spatial orientation.
Each p orbital consists of two lobes separated by a nodal plane (a region of zero electron probability) passing through the nucleus.
These orbitals appear first at the second energy level (n = 2). Hence, atoms like carbon and oxygen have 2p orbitals that play vital roles in bond formation and molecular geometry.
Orbital Capacity and Electron Spin
Orbital Occupancy
Orbital Capacity: Each atomic orbital can hold a maximum of two electrons.
This limit is governed by the Pauli Exclusion Principle, which states that no two electrons in an atom can have the same set of four quantum numbers. Therefore, when two electrons occupy the same orbital, they must have opposite spins (one with +½, the other with –½).
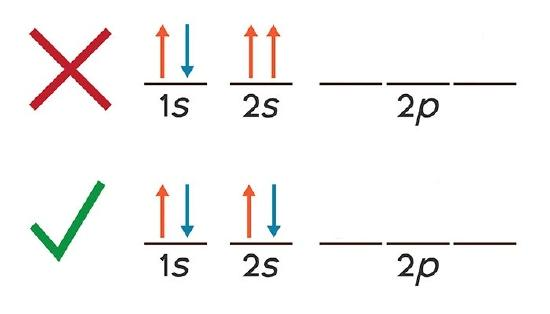
Pauli pairing in a single orbital. Two electrons can occupy one orbital only if their spins are opposite. The diagram emphasises the rule; any reference to specific subshell labels is incidental to the main concept. Source
This opposite spin pairing generates a small magnetic effect, effectively cancelling out magnetic fields and leading to electron stability within the orbital.
Relationship between Orbitals and Energy Levels
Each energy level (or shell) consists of one or more sub-shells, which contain orbitals of specific types and shapes.
For example:
First energy level (n = 1): Contains only one sub-shell — 1s.
Second energy level (n = 2): Contains 2s and 2p sub-shells (three p orbitals).
Third energy level (n = 3): Contains 3s, 3p, and 3d sub-shells (five d orbitals).
Fourth energy level (n = 4): Contains 4s, 4p, 4d, and 4f sub-shells.
In this subsubtopic, focus is limited to the s and p orbitals, but understanding their arrangement within these shells is essential for later topics on electron configuration and bonding.
Comparing s and p Orbitals
Key Differences
s Orbital: Spherical in shape, non-directional, and appears in every energy level. It has no nodal planes.
p Orbitals: Dumbbell-shaped, directional, and first appear at the second energy level. Each p orbital has one nodal plane through the nucleus and occurs in three orientations: px, py, and pz.
Physical Interpretation
The s orbital’s symmetry allows overlap in any direction, forming strong single (sigma) bonds.
The p orbitals enable directional bonding and formation of multiple bonds (sigma and pi bonds).
Understanding these distinctions is vital for predicting molecular shapes and bonding behaviour in organic and inorganic compounds.
Orbital Diagrams and Electron Density
Visualising orbitals helps in understanding how electrons occupy space within an atom.
s orbital: Appears as a sphere with uniform electron density.
p orbitals: Consist of two lobes on opposite sides of the nucleus; the electron density is concentrated in these lobes.
When drawing orbitals:
The nucleus is located at the centre.
The probability of finding an electron decreases farther from the nucleus.
Nodal planes (where electron density = 0) separate the lobes of p orbitals.
Energy and Spatial Distribution
The energy of an orbital depends mainly on the principal quantum number (n). Within the same shell:
s orbitals have lower energy than p orbitals because s electrons penetrate closer to the nucleus.
This penetration effect contributes to the energy ordering that determines orbital filling, explored in later subsubtopics.
Summary Points for OCR Focus
Atomic orbitals represent regions of high electron probability around the nucleus.
The s orbital is spherical and non-directional, while p orbitals are dumbbell-shaped and oriented along three axes.
Each orbital holds a maximum of two electrons with opposite spins.
s orbitals appear in all shells; p orbitals begin from the second shell.
Recognising the shapes and spatial orientations of these orbitals is essential for understanding bonding and molecular structure in further study of Chemistry.
FAQ
The size of an atomic orbital is mainly determined by the principal quantum number (n). As n increases, the orbital extends further from the nucleus, meaning the electron is more likely to be found at a greater average distance.
For example, a 2s orbital is larger than a 1s orbital, and a 3p orbital is larger than a 2p orbital. This increase in size also leads to higher energy, as the electron experiences less attraction to the nucleus.
p orbitals have angular nodes (nodal planes) because their wavefunctions change sign across the nucleus, creating regions where the probability of finding an electron is zero.
s orbitals, however, are spherically symmetrical, and their wavefunctions do not change sign with direction. Therefore, they have no nodal planes, though higher-energy s orbitals (e.g. 2s, 3s) can have radial nodes, where the probability temporarily drops to zero as distance increases from the nucleus.
Quantum numbers define each orbital precisely:
n (principal quantum number): Determines the size and energy level.
l (angular momentum quantum number): Defines the shape (s, p, d, or f).
ml (magnetic quantum number): Specifies orientation in space (e.g. px, py, pz).
For p orbitals (l = 1), ml can take three values (–1, 0, +1), producing three orientations along the Cartesian axes.
The Pauli Exclusion Principle states that no two electrons in the same atom can have identical sets of four quantum numbers.
Since an orbital already defines n, l, and ml, the only remaining variable is spin (ms). Thus, only two electrons can share the same orbital—one with spin +½ and the other with spin –½—ensuring unique quantum states for both.
In an s orbital, the probability density is highest near the nucleus and decreases uniformly in all directions, giving it spherical symmetry.
In a p orbital, the probability is concentrated in two lobes on opposite sides of the nucleus, separated by a nodal plane where the probability is zero.
This distribution affects how orbitals overlap during bonding, influencing molecular shapes and bond types.
Practice Questions
State what is meant by an atomic orbital and describe the shape of an s orbital. (2 marks)
1 mark for stating that an atomic orbital is a region around the nucleus where there is a high probability of finding an electron.
1 mark for correctly describing the shape of an s orbital as spherical (accept “sphere-shaped” or “round” for this mark).
Explain the differences between s and p orbitals in terms of their shape, energy, and orientation. Include examples of where each type of orbital is first found. (5 marks)
1 mark for stating that s orbitals are spherical and non-directional.
1 mark for stating that p orbitals are dumbbell-shaped and directional.
1 mark for mentioning that p orbitals are arranged along three perpendicular axes (px, py, pz).
1 mark for noting that p orbitals first appear in the second energy level, while s orbitals appear in all energy levels.
1 mark for explaining that p orbitals have slightly higher energy than s orbitals in the same shell due to less penetration towards the nucleus.

