OCR Specification focus:
'State how many electrons can fill the first four shells and relate this to energy levels.'
Introduction
Atoms contain electrons arranged in energy levels known as electron shells. Understanding the capacity of each shell and how it relates to atomic energy structure forms a foundation for explaining bonding, ionisation, and reactivity in chemistry.
Electron Shells and Energy Levels
Atoms have discrete energy levels surrounding the nucleus, often visualised as concentric shells where electrons reside.
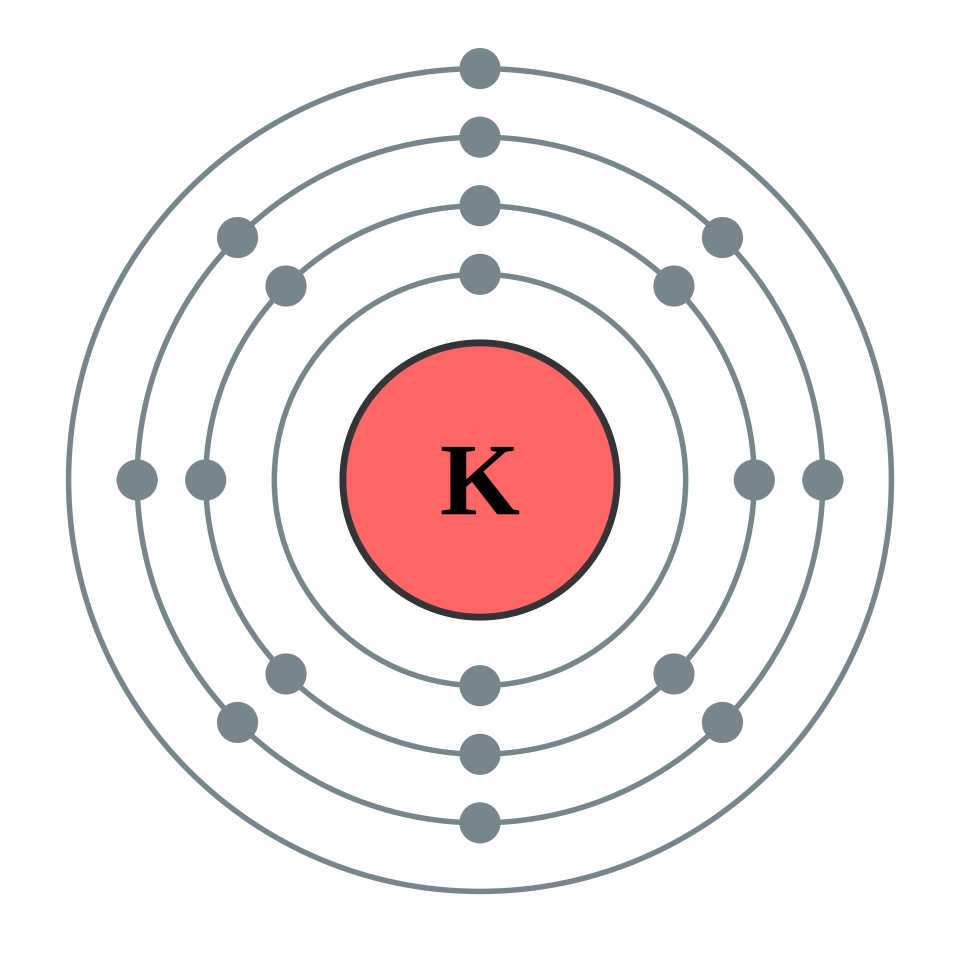
Bohr-style diagram of a potassium atom showing four concentric electron shells (n = 1–4). It illustrates how electrons occupy shells as principal energy levels; occupancies shown are for potassium and not the maximum shell capacities. Source
Definition of an Electron Shell
Electron Shell: A principal energy level within an atom where electrons with similar energies are likely to be found.
Each shell is identified by a principal quantum number, denoted as n = 1, 2, 3, 4, etc. The value of n determines both the average distance of electrons from the nucleus and the energy of the electrons within that shell.
Relationship Between Shell Number and Energy
The energy of electrons increases as the shell number increases.
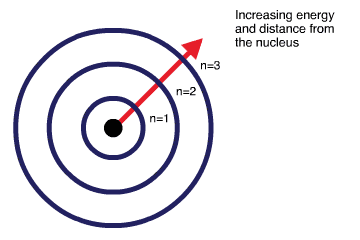
Diagram indicating that electron energy increases with distance from the nucleus, aligning shell number (n) with higher energy levels. This directly supports the link between shells and energy required by the specification. Source
The number of electrons that each shell can accommodate follows a simple relationship with the principal quantum number.
Electron Capacity of Shells
The maximum number of electrons in each shell is given by the formula:
Electron capacity of a shell = 2n²
n = Principal quantum number (1, 2, 3, 4, etc.)
This relationship defines how many electrons each of the first four shells can hold:
1st shell (n = 1): 2 × 1² = 2 electrons
2nd shell (n = 2): 2 × 2² = 8 electrons
3rd shell (n = 3): 2 × 3² = 18 electrons
4th shell (n = 4): 2 × 4² = 32 electrons
Thus, the electron capacities for the first four shells are 2, 8, 18, and 32 respectively.
Linking Electron Capacity to Energy Levels
The increasing number of electrons in higher shells corresponds directly to increased energy and complexity within the atom.
The first shell (n = 1) can hold only 2 electrons, forming the simplest configuration seen in hydrogen (1 electron) and helium (2 electrons).
The second shell (n = 2) holds up to 8 electrons, seen in elements like oxygen and neon.
As shells increase, they contain more sub-shells (s, p, d, f), allowing more electrons to occupy them.
Although the third shell can theoretically hold 18 electrons, in many lighter elements, it is not fully filled before electrons begin to populate the fourth shell due to differences in sub-shell energies. This is explored in later subtopics on orbital filling.
The Structure of Energy Levels
Each principal shell contains sub-shells, which represent finer divisions of energy within the shell. While this subsubtopic does not focus on sub-shell structure, it is essential to recognise that the total capacity of each shell arises from the number and type of these sub-shells (s, p, d, and f).
Increasing Energy and Shell Distance
Electrons in lower shells experience a stronger electrostatic attraction to the positively charged nucleus, giving them lower potential energy.
Electrons in higher shells are further from the nucleus, experiencing weaker attraction and therefore possessing higher potential energy.
As a result, when electrons transition between shells, energy is absorbed or released in quantised amounts, forming the basis of atomic emission and absorption spectra.
Electron Shells and the Periodic Table
The arrangement of electrons into shells is reflected in the structure of the Periodic Table.
Period number corresponds to the highest occupied shell (principal quantum number n).
For example:
Elements in Period 1 have electrons only in the 1st shell (n=1).
Elements in Period 2 have electrons in n=1 and n=2 shells.
Elements in Period 3 have electrons up to n=3, and so on.
This correspondence helps explain recurring patterns in element properties such as ionisation energy, atomic radius, and reactivity.
Energy Transitions Between Shells
Electrons can move between energy levels by absorbing or emitting specific amounts of energy, known as quantised energy.
Quantised Energy: Energy that exists in fixed, discrete amounts rather than a continuous range.
When an atom absorbs energy (for example, from heat or light), an electron may jump to a higher energy level (excited state). When it loses energy, the electron falls back to a lower energy level (ground state), releasing energy often as light.
These transitions produce distinct spectral lines, unique to each element, and provide experimental evidence for the existence of discrete energy levels within atoms.
Summary of Shell Capacities and Energy Levels
Key relationships between shell number, electron capacity, and energy:
Each shell (energy level) corresponds to a principal quantum number (n).
Electron capacity follows the formula 2n².
Energy increases with increasing shell number.
Electrons in outer shells are less strongly attracted to the nucleus and are more easily lost or shared during chemical reactions.
In the context of the OCR A-Level Chemistry specification, students must be able to state the electron capacities of the first four shells (2, 8, 18, 32) and understand how these values relate to increasing energy levels within atoms.
Key Points Recap
Electrons occupy discrete energy levels called shells.
Each shell has a principal quantum number (n) determining its energy and capacity.
The maximum number of electrons in each shell is 2n².
The first four shells can hold 2, 8, 18, and 32 electrons respectively.
Higher shells are further from the nucleus and have higher energy.
The Periodic Table reflects electron shell structure across periods.
Energy transitions between shells produce quantised spectral lines that confirm the existence of discrete energy levels.
FAQ
The 2n² rule arises from quantum mechanics. Each principal energy level (n) contains sub-shells, and each sub-shell has a set number of orbitals:
s = 1 orbital
p = 3 orbitals
d = 5 orbitals
f = 7 orbitals
Each orbital holds 2 electrons, giving the total electron capacity per shell as 2n². This relationship stems from the number of orbitals available within that energy level.
Electrons fill orbitals based on energy, not purely by shell number.
Some higher-energy sub-shells (like 4s) fill before lower-energy ones (like 3d).
This overlap causes irregularities in the expected filling sequence. Thus, while the theoretical capacity follows 2n², actual electron configurations depend on sub-shell energies.
When an electron moves from a higher to a lower energy level, it releases a photon of light.
The energy difference between shells determines the wavelength of that light.
Larger energy gaps (inner shells) produce light of higher energy (shorter wavelength). This process forms the emission spectra, which are unique to each element.
Evidence comes from atomic emission and absorption spectra.
Elements emit light at specific wavelengths, not a continuous spectrum.
Each line corresponds to an electron transition between fixed energy levels. This quantised behaviour confirms that electrons occupy discrete shells rather than a continuum of energies.
Atomic size increases with more shells, as outer electrons are farther from the nucleus.
Reactivity changes depending on how easily outer electrons are lost or gained.
In metals, more shells mean weaker attraction, so electrons are lost more easily.
In non-metals, outer shells farther away reduce the ability to attract new electrons.
Practice Questions
Explain how the number of electrons that can occupy an electron shell is related to its principal quantum number (n).
In your answer, you should:
State the formula used to determine the maximum number of electrons in a shell
Apply the formula to the first four shells
Describe how these shells relate to increasing energy levels in the atom
(5 marks)
States the correct relationship: Maximum number of electrons = 2n² – 1 mark
Applies the formula correctly to the first shell (2 × 1² = 2 electrons) – 1 mark
Applies the formula correctly to the second shell (2 × 2² = 8 electrons) – 1 mark
Applies the formula correctly to the third and fourth shells (18 and 32 electrons respectively) – 1 mark
Describes that as n increases, the shell is further from the nucleus and at a higher energy level – 1 mark
Total: 5 marks
State the maximum number of electrons that can occupy:
(a) the third electron shell
(b) the fourth electron shell
(2 marks)
(a) 18 electrons – 1 mark
(b) 32 electrons – 1 mark
Total: 2 marks

