OCR Specification focus:
‘Deduce electron configurations of atoms up to Z = 36 using sub-shell notation, e.g. oxygen as 1s2 2s2 2p4.’
Electron configurations describe how electrons occupy sub-shells within atoms. Understanding sub-shell notation, filling order and electron distribution up to Z = 36 is essential for interpreting structure, reactivity and periodic trends.
Electron Configuration and Sub-Shell Notation
Electron configuration expresses the arrangement of electrons across energy levels, sub-shells and orbitals. Sub-shell notation follows the structure nℓx, where n is the principal quantum number, ℓ is the sub-shell type (s, p, d) and x is the number of electrons in that sub-shell.
Electron configuration: The representation showing how electrons are distributed among an atom’s energy levels and sub-shells.
Each sub-shell contains a fixed number of orbitals, and each orbital holds up to two electrons with opposite spins. These restrictions determine how electrons fill in atoms up to Z = 36.
The Structure of Energy Levels and Sub-Shells
Atoms with atomic number ≤ 36 (from hydrogen to krypton) use the first four energy levels, each containing specific sub-shells:
1st shell: 1s
2nd shell: 2s, 2p
3rd shell: 3s, 3p, 3d
4th shell: 4s, 4p (4d is not required for atoms up to Z = 36)
Sub-shell Capacities
Each sub-shell has a fixed number of orbitals:
s-sub-shell: 1 orbital → holds 2 electrons
p-sub-shell: 3 orbitals → holds 6 electrons
d-sub-shell: 5 orbitals → holds 10 electrons
These values help determine maximum electron population in progressively larger atoms.
Filling Order: Increasing Energy and the Aufbau Principle
Electron configurations follow the Aufbau principle, which states that electrons fill sub-shells in order of increasing energy. For Z ≤ 36, this order is:
1s → 2s → 2p → 3s → 3p → 4s → 3d → 4p
Because the 4s sub-shell is slightly lower in energy than 3d in isolated atoms, it fills before 3d. Understanding this sequence is essential when deducing configurations.
Hund’s Rule and Pauli Exclusion
Two key rules also govern electron placement:
Hund’s rule: Electrons occupy orbitals singly before pairing, keeping spins parallel where possible.
Pauli exclusion principle: No two electrons in an atom can have the same set of quantum numbers; hence each orbital holds a maximum of two electrons with opposite spins.
These rules explain patterns such as the distribution of electrons in p-sub-shells and the filling of 3d orbitals.
Writing Electron Configurations in Sub-Shell Notation
Electron configurations list each occupied sub-shell in sequence with its electron count as a superscript (written here as a normal number).
General Guidelines
When deducing electron configurations up to Z = 36:
Identify the atomic number (Z) to determine the number of electrons.
Fill sub-shells following the established energy order.
Apply Hund’s rule in multi-orbital sub-shells.
Use sub-shell notation, e.g. 1s2 2s2 2p6.
Key Patterns in the First Four Periods
Electron configurations of elements up to krypton show characteristic patterns:
Periods 1 and 2: Fill 1s then 2s, followed by the three 2p orbitals.
Period 3: After 3s and 3p fill, the next sub-shell to be occupied is 4s.
Period 4: The 4s sub-shell fills before 3d; after 3d fills, electrons occupy 4p.
These patterns enable students to quickly identify the correct notation for any atom in this range.
Using this pattern, the sub-shells for atoms up to krypton fill in the order 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p.
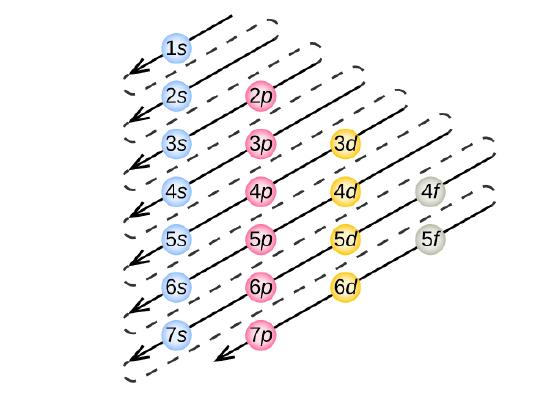
Diagram showing the order of filling of atomic subshells according to the Aufbau principle. Each diagonal arrow indicates the sequence in which electrons occupy subshells, beginning from 1s. Higher subshells (4d, 5f) appear but extend beyond the OCR requirement while following the same logic. Source
Full Sub-Shell Occupancy and Noble Gas Structure
The noble gases (He, Ne, Ar, Kr) demonstrate complete outer shells or sub-shells, offering useful reference points:
He (Z = 2): 1s2
Ne (Z = 10): 1s2 2s2 2p6
Ar (Z = 18): 1s2 2s2 2p6 3s2 3p6
Kr (Z = 36): 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6
The stability of these configurations supports trends in reactivity and periodicity.
Special Cases and Stability Considerations
Although most configurations follow the predicted order, a few atoms show minor deviations due to increased stability from half-filled or fully filled d-sub-shells. For Z ≤ 36, the most relevant considerations occur in the 3d block, where electron repulsion and sub-shell energies influence final arrangements. However, the OCR specification for this sub-subtopic requires only that students deduce configurations using the standard order without additional exceptions.
Bullet-Point Checklist for Students
To deduce electron configurations effectively:
Determine the number of electrons (equal to Z for neutral atoms).
Apply the filling order from 1s to 4p.
Remember: 4s fills before 3d.
Use sub-shell notation with superscripts indicating electron count.
Apply Hund’s rule for p and d sub-shells.
Verify that total electrons match the atomic number.
Relation to Chemical Behaviour
Electron configuration influences chemical properties such as reactivity, ionisation energy and bonding tendencies. Elements with similar outer electron configurations, particularly those in the same group, exhibit comparable chemical behaviour. Understanding configurations up to Z = 36 allows students to recognise the foundation of periodic trends, predicting behaviour of s-, p- and early d-block elements with confidence.
For any neutral atom, the total electrons in all its sub-shells must add up to the atomic number, and these electrons are distributed across shells that correspond to the principal quantum numbers.
Periodic table annotated with the number of electrons in each shell for every element. For atoms up to krypton, the shell distributions shown match the electron configurations discussed in this section. Elements beyond Z = 36 are included, which is additional information not required by OCR but consistent with the same shell-filling rules. Source
FAQ
The relative energies of 4s and 3d change once electrons occupy the atom. Before filling, 4s is slightly lower in energy, so electrons enter 4s first.
After 3d begins to fill, electron–electron repulsion shifts the 4s sub-shell to slightly higher energy than 3d.
As a result, 4s electrons are removed first during ion formation, particularly in transition metals.
The block in which an element sits indicates its final sub-shell:
s-block: Groups 1 and 2 (plus helium)
p-block: Groups 13–18
d-block: Transition metals (periods 4–5 for this subsubtopic)
For elements up to Z = 36, krypton ends in 4p, zinc ends in 3d, and calcium ends in 4s.
Sub-shells with symmetrical electron arrangements are slightly more stable.
This stability arises from reduced electron–electron repulsion and increased exchange energy.
For example, a half-filled p sub-shell (p3) distributes electrons evenly across the three orbitals.
Although OCR does not examine exceptions in detail here, the concept explains minor deviations in transition-metal configurations.
Orbital diagrams provide a visual representation of electron placement.
They show:
Each orbital as a box
Electrons as arrows with opposite spins
Application of Hund’s rule in multi-orbital sub-shells
Sub-shell notation summarises this information without diagrams, condensing the electron distribution into a written sequence such as 2p4.
Electron configuration determines both the period and the group of an element.
The highest principal quantum number containing electrons indicates the period.
The number of electrons in the outer sub-shell (s or p) determines the group for main-group elements.
Up to Z = 36, this relationship allows accurate placement of every element from hydrogen (1s1) to krypton (4p6).
Practice Questions
Write the electron configuration for the element phosphorus (Z = 15) using full sub-shell notation.
State the number of electrons in the 3p sub-shell.
(2 marks)
1s2 2s2 2p6 3s2 3p3 (correct full electron configuration) — 1 mark
3 electrons in the 3p sub-shell — 1 mark
Krypton (Z = 36) is the last element for which you must be able to write a full electron configuration in this topic.
(a) Deduce the full electron configuration of krypton.
(b) Explain why the 4s sub-shell fills before the 3d sub-shell, even though 4s has a higher principal quantum number.
(c) Using Hund’s rule, describe how electrons fill the 3p sub-shell in an atom.
(5 marks)
(a)
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 — 1 mark
(b)
4s is lower in energy than 3d in isolated atoms — 1 mark
Therefore 4s fills before 3d according to the Aufbau principle — 1 mark
(c)
Electrons occupy orbitals singly before any pairing occurs — 1 mark
Electrons in singly occupied orbitals have parallel spins — 1 mark
Total = 5 marks

