OCR Specification focus:
‘Describe orbital filling: increasing energy order for 1s to 4p; orbitals of equal energy are singly occupied before pairing.’
Introduction
The arrangement of electrons within atoms follows defined principles that ensure electrons occupy the lowest-energy positions available while maintaining maximum stability. This subtopic explains how orbitals fill in increasing energy order and how Hund’s rule determines electron distribution within degenerate orbitals.
The Concept of Orbital Filling
Electron configuration describes how electrons are arranged within atomic orbitals, following a predictable sequence based on increasing energy. Understanding this order is essential for explaining patterns in the Periodic Table and the reactivity of different elements.
Increasing Energy Order from 1s to 4p
Electrons fill orbitals in a sequence determined by orbital energy, with lower-energy orbitals filling before higher-energy ones. This sequence applies to all atoms and ensures that the most stable configuration is achieved.
The increasing energy order of sub-shells needed for OCR is 1s < 2s < 2p < 3s < 3p < 4s < 3d < 4p.
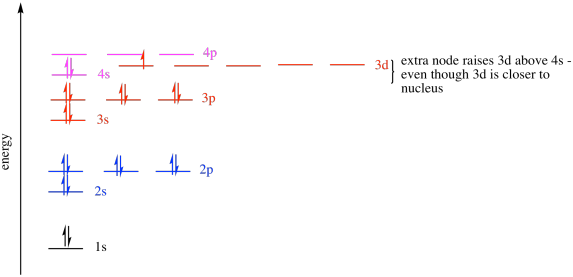
This orbital energy diagram shows the relative energies of the 1s, 2s, 2p, 3s, 3p, 4s, 3d and 4p sub-shells, with energy increasing up the vertical axis. It illustrates that the 4s orbital lies slightly lower in energy than the 3d orbitals, explaining why 4s fills before 3d. The diagram also includes electrons in each orbital, which exceeds OCR requirements but helps students relate energy ordering to occupied orbitals. Source
The Energy Levels and Sub-shell Structure
Each principal energy level contains sub-shells (s, p, d, f), each with a specific number of orbitals. Understanding how these sub-shells relate to electron filling provides structure to electron configuration.
Orbital: A region around the nucleus that can hold up to two electrons with opposite spins.
Orbitals within the same sub-shell (for example, the three p orbitals) have the same energy and are known as degenerate orbitals. The way electrons occupy these affects atomic behaviour and magnetic properties.
Between major energy levels, there is overlap, meaning energy values do not increase evenly. This is why the 4s orbital fills before the 3d orbital, even though 4s is in a higher principal energy level.
Electron Spin and Pairing Principles
Electrons possess a property called spin, described as either ‘up’ or ‘down’. When two electrons occupy the same orbital, they must have opposite spins to minimise repulsion.
Spin: An intrinsic property of electrons that can exist in one of two opposite orientations.
Orbital filling rules use spin to maintain stable arrangements and minimise electron–electron repulsion. These rules underpin the formation of the electron configurations of atoms.
A single sentence to separate definition blocks ensures clarity in the structure of explanations.
Hund’s Rule and Its Importance in Electron Filling
Hund’s rule is fundamental when filling orbitals of equal energy within the same sub-shell. The rule ensures electrons remain unpaired where possible to maintain a lower-energy configuration.
Hund’s Rule: Electrons occupy orbitals singly with parallel spins before any pairing occurs in orbitals of the same energy.
Hund’s rule applies only to degenerate orbitals, such as the three p orbitals or five d orbitals. It ensures that electron repulsion is minimised by spreading electrons out. As a result, atoms with half-filled or fully filled sub-shells often display increased stability.
Applying Hund’s Rule to Sub-shells
When filling p or d sub-shells, electrons follow a specific distribution pattern:
Electrons occupy each orbital singly before pairing begins.
All single electrons in degenerate orbitals have parallel spins.
Pairing occurs only once all orbitals contain one electron.
This leads to characteristic patterns in electron configuration diagrams. For example:
The p sub-shell (three orbitals) can hold up to six electrons.
According to Hund’s rule:
The first three electrons occupy different p orbitals singly.
The next three electrons pair up to complete the sub-shell.
Following this pattern helps predict magnetic behaviour—species with unpaired electrons often show paramagnetism.
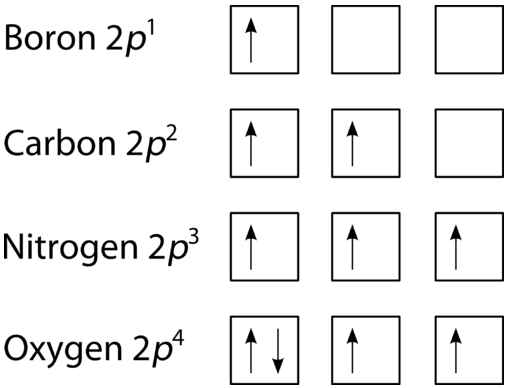
This diagram shows the three 2p orbitals for boron, carbon, nitrogen and oxygen, with arrows representing electron spins. It demonstrates how each orbital is singly occupied with parallel spins before any pairing occurs, directly illustrating Hund’s rule. The inclusion of specific elements goes beyond OCR requirements but helps students see how the rule functions in real electron configurations. Source
The Aufbau Principle and Filling Order
Hund’s rule works alongside the general principle governing how orbitals fill: the Aufbau principle, which states that electrons occupy the lowest-energy orbital available first.
Aufbau Principle: Electrons fill orbitals starting with the lowest-energy level before occupying higher-energy orbitals.
The combination of Hund’s rule and the Aufbau principle produces the characteristic electron configurations used throughout A-Level Chemistry.
A brief sentence between definition blocks keeps the flow of information smooth and consistent with required formatting.
Key Rules for Determining Electron Configuration
To use orbital filling order and Hund’s rule effectively, remember the core principles that govern electron arrangement:
Essential Orbital Filling Rules
Lower-energy orbitals fill before higher-energy ones.
The 4s orbital fills before the 3d orbital for neutral atoms.
Each orbital holds a maximum of two electrons with opposite spins.
Orbitals in the same sub-shell are filled singly before pairing (Hund’s rule).
Electrons remain unpaired for as long as possible to minimise repulsion.
Electron configurations follow the pattern dictated by energy order from 1s to 4p.
Benefits of Understanding Filling Patterns
Understanding electron filling allows students to:
Predict stable electron configurations.
Recognise unusual configurations such as those involving transition metals.
Explain magnetic and chemical properties of atoms.
Interpret patterns in the Periodic Table linked to sub-shell structure.
FAQ
In neutral atoms, the 4s orbital is slightly lower in energy, so it fills first.
However, once electrons begin occupying the 3d sub-shell, increased electron–electron repulsion raises the energy of the 4s orbital relative to 3d.
As a result, in many transition metal ions, the 4s electrons are removed before the 3d electrons.
Parallel spins minimise repulsion because electrons with the same spin tend to avoid each other spatially.
This spreads electrons across orbitals more effectively.
Lower repulsion leads to lower overall energy
Lower energy corresponds to greater stability
No. Most exceptions arise from very small energy differences between sub-shells, not from breaking Hund’s rule.
Elements like chromium and copper follow Hund’s rule inside the 3d sub-shell; their exceptions occur due to the stability of half-filled or fully filled d sub-shells.
Hund’s rule determines the number of unpaired electrons in an atom.
Atoms with unpaired electrons are paramagnetic
Atoms with all electrons paired are diamagnetic
Thus, the distribution of electrons across degenerate orbitals predicts whether an atom is attracted or repelled by a magnetic field.
Pairing electrons introduces additional repulsion due to their like charges.
Occupying separate degenerate orbitals reduces this repulsion.
Single occupation is always lower in energy than pairing
Pairing becomes favourable only once all orbitals contain one electron
This principle underpins Hund’s rule across all p, d, and f sub-shells.
Practice Questions
State the order in which the following orbitals are filled in a neutral atom: 3p, 4s, 3d.
Explain briefly why the 4s orbital fills before the 3d orbital.
(2 marks)
1 mark: Correct order stated as 3p → 4s → 3d.
1 mark: Explanation that 4s is lower in energy than 3d in neutral atoms.
Using Hund’s rule and the orbital filling order, describe and explain how the five 3d orbitals are filled in a transition metal atom.
In your answer:
State the rule governing how electrons occupy orbitals of equal energy.
Explain why electrons remain unpaired where possible.
Describe how many electrons can occupy the 3d sub-shell and how they fill across the five orbitals.
(5 marks)
1 mark: States Hund’s rule (orbitals of equal energy are singly occupied before pairing).
1 mark: Explains this reduces electron–electron repulsion or increases stability.
1 mark: States that the 3d sub-shell contains five orbitals.
1 mark: States that each orbital can hold a maximum of two electrons.
1 mark: Describes correct filling pattern: one electron enters each of the five 3d orbitals with parallel spins before any pairing occurs.

